Discover how chemical reactions keep people alive when they reach for extra oxygen
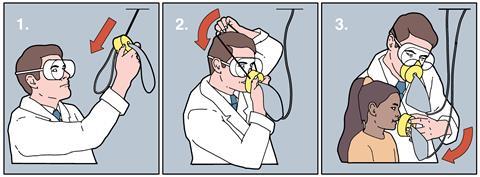
‘In the event of loss of cabin pressure during today’s flight, oxygen masks will fall from the overhead panel.’ If you have flown before, you’ll be familiar with this phrase. But have you ever considered where the oxygen that flows through those masks comes from?
It might surprise you to learn that, for most passenger aircraft, the answer is a chemical reaction. A small metal cylinder-shaped container is located above each row of seats and the masks are attached to these. When a passenger tugs a mask, a firing pin is pulled from the cylinder. This action triggers a tiny explosion that produces the energy needed to start a chemical reaction inside the container, generating oxygen.
In your class
Discuss atmospheric components to reinforce curriculum-based learning about atoms, elements, molecules and compounds. Give learners the opportunity to describe the subtle differences between molecules, such as oxygen and carbon dioxide, to check their understanding and address misconceptions.
These devices are known as chemical oxygen generators and they produce oxygen for around 15 minutes – enough time for a pilot to drop the plane to an altitude where supplementary oxygen is no longer needed.
Chemical oxygen generators ‘generate molecular oxygen by chemical means’, explains Richard Imbruce, chief executive officer at Rapid Oxygen Company in Connecticut, US. The oxygen source in these devices is usually a chlorate or perchlorate that decomposes when heated to produce oxygen gas. Sodium chlorate, for example, decomposes above 300°C, to release oxygen and form sodium chloride (a white solid). ‘It’s an exothermic reaction that produces a lot of heat,’ Richard says. The resulting burning smell can add to the panic of the situation.
Lifesavers
The chemicals in most of these devices are highly stable and can therefore be stored almost indefinitely until needed. Due to their long shelf life and small size, these devices are well suited for a range of emergency applications beyond flying, including as emergency backup oxygen supplies in submarines and on the International Space Station.
Rapid Oxygen Company’s chemical oxygen generator is called the R15, and so far it has been placed in homes, manufacturing facilities, shopping malls, schools, hotels, airports and sports-fitness programs across the US. The R15 is typically installed next to a defibrillator, to create an emergency kiosk. ‘The whole idea is getting oxygen [to the patient] as soon as possible,’ says Richard. ‘After just two minutes without oxygen the brain degenerates, much shorter than the 8-minute average emergency medical services response time in the US.’ The R15 generates oxygen for a minimum of 15 minutes, enough time for the emergency services to arrive with additional oxygen.
Download this
Quiz, for age range 11–14
Use this quiz to reinforce learning about the differences between atoms, molecules, elements and compounds.
Download the resources from the Education in Chemistry website: rsc.li/3gc1mIH
The R15 is a next-generation chemical oxygen generator that uses sodium percarbonate as its oxygen source, which decomposes at a lower temperature (180°C) than the chemicals used in most chemical oxygen generators. The activation mechanism is also mechanical rather than explosive. As a result, Richard describes the R15 as ‘a safe, low-pressure method to produce emergency oxygen’.
Accidents caused by traditional-style chemical oxygen generators are rare, but not unheard of. In 1996, expired and poorly-stored chemical oxygen generators caused a fire in the cargo hold of ValuJet Airlines Flight 592, causing the plane to crash into the Florida Everglades, killing all 110 passengers on board. In 2007, two mechanics died when a poorly stored and maintained chemical oxygen generator exploded on the nuclear submarine HMS Tireless.
Separation successes
The oxygen gas in cylinders, such as those used in hospitals and by paramedics, divers and high altitude climbers, is produced by a different method: the fractional distillation of air. Ambient air contains around 78% nitrogen, 21% oxygen and 1% other gases including carbon dioxide and water vapour. The ambient air is collected and then cooled down to -200°C; oxygen liquefies at -183°C and nitrogen at -196°C. This liquid air is then warmed up slowly in a fractional distillation column, which separates the gases based on their boiling points. Oxygen cylinders are for prolonged use.
Another tool for generating oxygen gas is the oxygen concentrator. As its name implies, these devices do not generate 100% oxygen, but rather concentrate the oxygen in ambient air to about 93% oxygen. This oxygen-rich gas is then administered to patients via a nasal cannula. ‘These devices are used mostly for patients with chronic obstructive lung disease in their homes,’ explains Richard. Their sizes range from that of a small suitcase to portable devices that can be easily carried on your person. ‘Oxygen concentrators function by compressing ambient air to increase a pressure gradient through a molecular sieve that separates out nitrogen – a larger molecule – and concentrates oxygen,’ Richard says.
Remember, if you ever find yourself in need of emergency oxygen – no matter where you are in the world (or even the universe) – chemistry can come to your rescue.
More resources
- Read about how electrochemistry is being used to secure astronauts’ oxygen on the Moon.
- Show the formation of elements and compounds in your 11–14 classes using these demonstrations, practicals and modelling activities.
- Boost 11–14 student understanding of atoms, molecules and ions with these ideas and resources.
- Refresh your understanding of classroom modelling with the RSC on-demand developing and using models CPD course.
More resources
- Start an engaging class discussion on how electrochemistry is being used to secure astronauts oxygen on the Moon: rsc.li/3VHOIS9
- Show the formation of elements and compounds in your 11–14 classes using these demonstrations, practicals and modelling activities: rsc.li/3eIcgWv
- Boost 11–14 student understanding of atoms, molecules and ions with these ideas and resources: rsc.li/3TjNYB8
- Refresh your understanding of classroom modelling with the RSC on-demand developing and using models CPD course: rsc.li/3VJjFFl














No comments yet