In the July 2012 exhibition chemistry article we looked at Field’s metal, which melts at a surprisingly low temperature of 61°C. This mixture of indium, bismuth and tin is great for showing how an alloy will have different properties from its constituents. Another such alloy is a mixture of sodium and potassium, and it displays a fascinating property at room temperature that students really don’t expect.
Kit
- Sodium (releases flammable gases in contact with water, corrosive)
- Potassium (releases flammable gases in contact with water, corrosive)
- 100 cm3 propan-2-ol (highly flammable, eye irritant)
- 100 cm3 hydrocarbon solvent* (highly flammable)
- 10 cm3 butan-1-ol (flammable,corrosive to eyes, irritant to skin and respiratory system, harmful if swallowed)
- Two 125 × 16 mm borosilicate test tubes
- Test tube rack
- Knife and two petri dishes
- Metal spatula
- Paper towel
- Tweezers
- Dropping pipette (that reaches to the bottom of the test tubes)
*Cyclohexane, petroleum ether (bp 100–120°C) or kerosene are suitable for this demonstration. For safety reasons, avoid low boiling point solvents. Cyclohexane is highly flammable, and a skin and respiratory irritant. Petroleum ethers are highly flammable and carcinogenic. All three are aspiration toxins.
Preparation
Ensure all equipment is completely dry. Cut one piece of sodium and four pieces of potassium all about the same size – aim for rough cubes with sides of 3–4 mm (mass < 0.1 g). Leave these separately under a layer of the solvent in the petri dish until ready to use.

In front of the audience
Add solvent to both test tubes to a depth of about 3 cm. Add the pieces of sodium and potassium to one of these. The metal will sit at the bottom of the liquid. Work with the tube in a rack to avoid spilling on your hand should the glass break. Use the spatula to chop up and mash together the pieces of metal. After about 30 seconds, the metal mixture will feel very soft and you may be able to see liquid metal among the surface oxides. Add two drops of butan-1-ol. This will assist the metal droplet in coalescing away from the insoluble impurities. Use the dropping pipette to draw up the liquid metal, leaving the rest of the impurities in the liquid, and add this droplet to the second test tube so it can be inspected.
Teaching goal
The sodium–potassium alloy, commonly known as NaK, is used most famously as a coolant in fast reactors. It has advantages over other coolants in the wide temperature range at which it remains liquid and its low tendency to absorb neutrons.
This demonstration lends itself well to teaching metallic bonding for 14–16 year-olds – such a model would refer to a lattice of positive metal ions that experience an electrostatic attraction to surrounding delocalised valance electrons (download classroom PowerPoint slides).
Students would be expected to connect the radius of the cation as well as its charge and number of delocalised electrons to the melting point of the metal. Given potassium’s larger ionic radius, it’s not surprising to find it has a lower melting point than sodium. Students may predict any mixture of the two metals to have a melting point between that of the two components so it’s a pleasant surprise to see a liquid rather than a solid appear from mixing the two.
One way to explain this would be to use the enthalpy of mixing as a proxy for the strength of the forces between the particles in the sodium, potassium and in the mixture. For all mixtures of sodium and potassium, the enthalpy of mixing is endothermic, suggesting weaker forces between the particles in the mixture than between those in the pure substances.
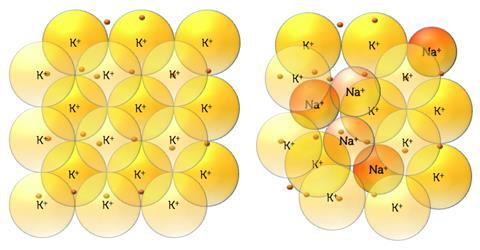
The enthalpy of mixing for mixtures of metals depends primarily on the crystal structures, any difference in electronegativity, valence and the ionic radiuses of the components. Both sodium and potassium have the same valance, face-centred-cubic structures and similar electronegativities, so this leaves the difference in the ionic radius as the primary culprit for the weaker forces observed.
The efficiency of packing cations in the mixture is poorer than that of either pure substance. This can be shown diagrammatically or by making model structures with marbles or coins.
Safety
- Wear goggles.
- When cutting up the Na and K, take care to replace the unused pieces in the correct bottle – replace one metal, before opening the next bottle.
- To dispose, draw off the paraffin and use the pipette to add the alloy in small drops to a beaker containing propan-2-ol. Wash the contents of both tubes and the propan-2-ol down the sink with plenty of water. Finally rinse out all the equipment with water in case any last specks of alkali metal remain.
- Do not attempt this demonstration with larger pieces of metal. This demonstration is unlikely to be covered by your school’s model risk assessments. Members of CLEAPSS and SSERC can obtain a special risk assessment by contacting them directly.
- Both solvents can cause dizziness/drowsiness if inhaled in sufficient quantities.
Downloads
Finding the NaK MS Powerpoint slides
PowerPoint, Size 7.82 mb



















No comments yet