Quiz your students’ on their knowledge of quantitative chemistry methods
The topics covered in this Starter for ten are: the mole; moles and mass; mass and concentration; concentration and dilution; moles summary; the ideal gas equation; molar gas volume; empirical and molecular formula; percentage yield and atom economy; and titration calculations.
Example questions
Work out the answers to the following simple calculations (1 t = 1 tonne = 1,000 kg);
- No. of moles in 10.0 g of O2 + the mass in g of 2.41 moles of H2O =
- Mass in g of 0.2 moles of K2CO3 + mass in g of 0.5 moles of MgCO3 =
- No. of moles in 12.4 t of NaNO3 ÷ no. of moles in 12.4 t of NaCl =
- No. of moles in 25.9 g of sodium – no. of moles in 25.9 g of sodium chloride =
- ? × molar mass of in g mol-1 of calcium carbonate = no. of moles in 4.2 kg of SiCl4
Calculate the answers to the calculations below and place them (to the correct no. of sig. fig.) in the appropriate square. The arrows indicate the direction the numbers must follow. For the 10th mark complete the remainder of the Sudoku grid.
WARNING Take care with your significant figures and RAMs in order to avoid the wrong digit in the wrong square! (Relative atomic masses, H 1.0; O 16.0; Na 23.0; S 32.1; Cl 35.5; Fe 55.8; Cu 63.5)
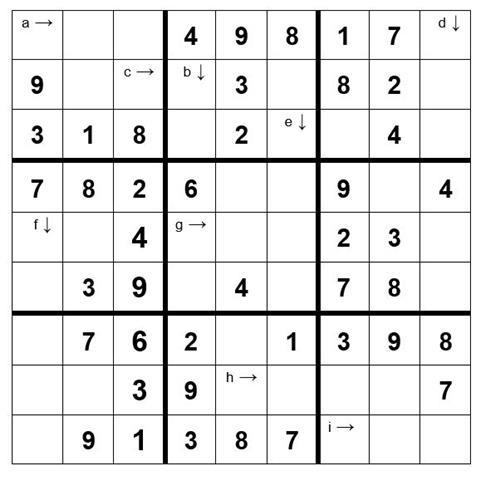
- The concentration of a solution of 265 moles of NaOH dissolved in 1 dm3 of water (3 sig. fig.)
- The volume of water in dm3 needed to dilute 176 moles of HCl to make a 1 mol dm-3 solution (3 sig. fig.)
- The mass of H2SO4 that should be dissolved in 1 dm3 of water to make a solution of concentration 0.72 mol dm-3 (2 sig. fig.)
- The volume of water in cm3 that must be added to 0.56 g of anhydrous CuSO4 to produce a 0.1 mol dm-3 solution (2 sig. fig.)
- The number of moles of ammonia that must be dissolved in 2,696 dm3 of water to produce 2.0 mol dm-3 ammonia solution (4 sig. fig.)
- The concentration in mol dm-3 of an accurate solution of concentration 16.48537 mol cm-3 (5 sig. fig.)
- The mass of FeSO4.7H2O that must be dissolved in 1,582 cm3 of water to form a solution of concentration 2.0 mol dm-3 (to 3 sig. fig.)
- The volume in dm3 of water that 10 moles of NaCl must be dissolved in to produce a 0.0155 mol dm-3 solution of brine (3 sig. fig.)
- The concentration in mol dm-3 of a solution of NaOH with a concentration of 18,480 kg m-3 (3 sig. fig.)
Notes
The full document, including questions and answers, are available from the ’Downloads’ section below. An editable version is also available.
Downloads
Quantitative chemistry - editable
Word, Size 0.78 mbQuantitative chemistry
PDF, Size 0.86 mb
Starters for 10: Advanced level 1 (16–18)
- 1
- 2
 Currently reading
Currently readingQuantitative chemistry
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12





























No comments yet