Kay Stephenson and Dorothy Warren explain common misconceptions and show you how you could help your students
Chemical bonding (and related ideas about chemical stability/reactivity) is acknowledged as being a ‘tricky to teach’ topic … with good reason! It is an abstract, theoretical idea that requires students to develop and apply increasingly sophisticated ‘molecular-scale’ models in order to make sense of their observations of the macroscopic properties of different substances.
As Keith Taber points out, students commonly acquire alternative conceptions (‘misconceptions’) about chemical bonding.1a,3 Some of these can be persistent and may present significant barriers to students’ progression and understanding of more complex ideas in chemistry. There are ways to find out if your students have these misconceptions, what might cause them and alternative teaching strategies that might alleviate them.
Chemical stability
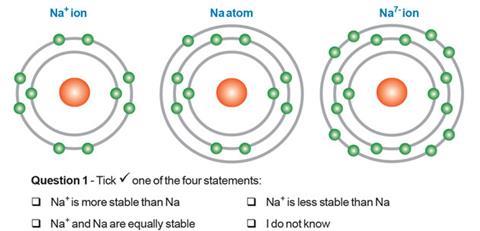
Significant proportions1b of pre-16 and post-16 students appear to develop the firm belief that ‘… species with octet configurations or full outer shells are always more stable than species with other configurations.’
You can find out what your students believe by using a diagnostic tool (see Chemical stability diagnostic).
We have learned from trials that 97% of 15–16 year olds believed the Na+ ion would be more stable than the neutral atom and around 80% thought the Na7– anion would also be more stable than the atom (as did a significant number of post-16 chemistry students in the trial!). As one teacher commented, ‘… it is clear that the complete shell of electrons dominates their thinking.’2a
Chemical bonding
Students often suggest that atoms need to acquire a full outer shell and think that this is the ‘driver’ for chemical reactions. For many students ‘ … this belief is so strong that they tend to offer this explanation even when they have been taught more appropriate ideas’.1,2
You could try a probe (see Why do hydrogen and fluorine react?) to find out what your students understand.2b
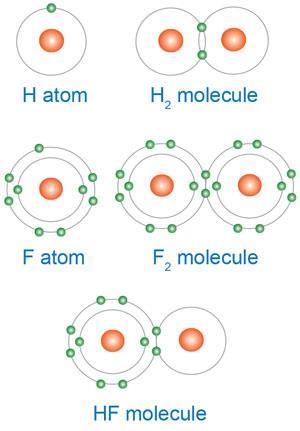
Post -16 students often still give explanations in terms of ‘happy’ atoms (ie full outer shells) even when they have studied relevant aspects of thermodynamics and are aware that the reacting elements exist as diatomic molecules (ie already have ‘full outer shells’!).1-3
Causes of misconceptions
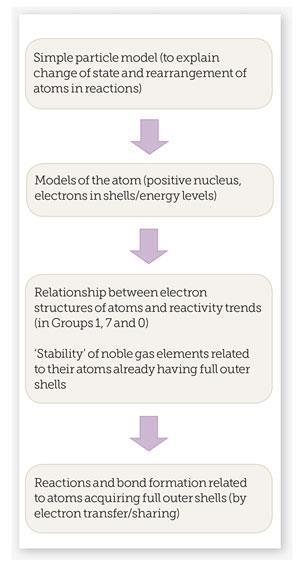
How do these misconceptions arise? If we look at the teaching ‘route’ that’s typically followed in pre-16 texts and published courses, we could find the answer.
It’s fairly easy to see how, without careful teaching, students could come to the conclusion that reactions happen because atoms are ‘trying’ to gain full outer shells and that there are just two ways of doing this, by:
- Transferring electrons to form ionic bonds.
- Sharing electrons to form covalent bonds.
Also, perhaps, awarding organisations’ guidance isn’t as helpful as it could be (see Misconceptions or required learning?). These statements are not quotations from confused students but from current GCSE specifications.
Misconceptions or required learning?
- [The noble gases] are unreactive because their atoms have stable arrangements of electrons.
- … chemical bonding involves either transferring or sharing electrons … in order to achieve the electronic structure of a noble gas.
- … a covalent bond is a shared pair of electrons.
- … ionic bonds are formed by the transfer of electrons to produce cations and anions.
Electrostatics approach
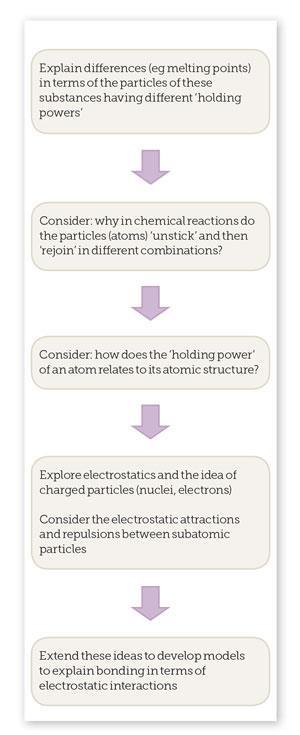
By shifting the focus at an early stage (pre-16) onto the electrostatic nature of chemical bonding, we could provide a more logical approach and a sound basis for students’ progression and facility in dealing with more complex ideas.
A good place to start would be to think about how useful the simple particle model is for explaining the behaviour of pure substances. You can explore your students’ ideas with some ‘simple’ (or not) demonstrations (see Demonstration suggestions).
Although our early particle model can explain some aspects, we need to develop more sophisticated models to explain all our observations.
Interestingly, although many of the examination specifications helpfully include the idea that our model of an ionic bond involves attraction between oppositely charged ions, few seem to extend this to explain that the key feature of the covalent bond model is the mutual attraction between the shared electrons and the nuclei. However, the latest version of the subject content and assessment criteria for GCSE Science (England)6 spells out that students should be able to ‘explain chemical bonding in terms of electrostatic forces’ – so, perhaps, we will see this reflected more explicitly in published assessment and teaching resources.
Students are familiar with electrostatic phenomena (balloons sticking to walls, hair standing on end) and the ‘invisible forces’ of attraction and repulsion and can apply these ideas to help them understand chemical bonding (see Analogy for the atom).
Although clearly not the whole picture, the electrostatics approach can support more effective progression and help students appreciate related aspects, such as: why some bonds are stronger, bond enthalpies, giant lattice structures v simple molecules, polar covalent bonds and intermolecular bonds.
Analogy for the atom
Try this exercise with your students; it is designed to probe and challenge their ideas about the forces acting in an atom.
Demonstration suggestions
More CPD
This topic is covered in one of the Royal Society of Chemistry’s Developing Expertise in Teaching courses. The RSC can support you throughout your teaching career.
Visit the CPD for Teachers webpage to find out more about the courses on offer.
Kay Stephenson and Dorothy Warren are both independent science education consultants based in the UK
References
- K S Taber, Chemical misconceptions – prevention, diagnosis and cure(Volume 1: theoretical background). Royal Society of Chemistry, 2002
- p125 (Chemical bonding)
- p85 (Chemical axioms)
- K S Taber, Chemical misconceptions – prevention, diagnosis and cure(Volume 2: Classroom resources). Royal Society of Chemistry, 2002
- p121 (Chemical stability)
- p107 (Hydrogen fluoride)
- K Taber (ed.), Teaching Secondary Chemistry (2nd ed.). Hodder Education, 2012 (especially chapters 1–4)
- V Kind, Beyond appearances: Students’ misconceptions about basic chemical ideas (2nd ed.). Royal Society of Chemistry, 2004 (pdf)
- Education in Chemistry, May 2011, p87 (Chemical bonding)
- Science: GCSE subject content and assessment objectives. Department for Education, 2013









1 Reader's comment