Understanding the risks will help your students practise chemistry safely – and apply those lessons to life too
David Paterson updated this article on 21 July 2025.
Why can there be such a mismatch between our perception of risk and the actual risk of an activity? The probability of dying in a plane crash is estimated at 1 in 11 million, while dying in a car crash is estimated at 1 in 5000. However, many more people are scared of flying than driving in a car.
Many people are happy to sunbathe or use a sunbed but worry about the effects of radiation therapy to treat disease. Even one sunbed session can increase the chance of some skin cancers by 20% or more, while radiation therapy is integral in treating cancers.

Over the years, when speaking with teachers and technicians I have heard phrases like ‘You can’t do that experiment. It’s too dangerous’; ‘That chemical is banned, so we don’t do that practical activity anymore’ and ‘I’m nervous that it might go wrong so I will put on a video instead.’ In reality, as long as teachers and students are confident with handling the apparatus and they have considered the risks involved (i.e. done a risk assessment), then they can carry out most practical activities safely in the lab. CLEAPSS has a useful guide on which chemicals are actually banned in school labs (very few) and which activities can’t be done (and safe alternatives to those that are).
Why can there be such a mismatch between our perception of risk and the actual risk of an activity? The probability of dying in a plane crash is estimated at 1 in 11 million, while dying in a car crash is estimated at 1 in 5000 (bit.ly/3GCYG4F). However, many more people are scared of flying than driving in a car.
Many people are happy to sunbathe or use a sunbed but worry about the effects of radiation therapy to treat disease. Even one sunbed session can increase the chance of some skin cancers by 20% or more, while radiation therapy is integral in treating cancers (bit.ly/46lqncB).
Over the years, when speaking with teachers and technicians I have heard phrases like ‘You can’t do that experiment. It’s too dangerous’; ‘That chemical is banned, so we don’t do that practical activity anymore’ and ‘I’m nervous that it might go wrong so I will put on a video instead.’ In reality, as long as teachers and students are confident with handling the apparatus and they have considered the risks involved (i.e. done a risk assessment), then they can carry out most practical activities safely in the lab. CLEAPSS has a useful guide (bit.ly/3TS06v8) on which chemicals are actually banned in school labs (very few) and which activities can’t be done (and safe alternatives to those that are).
The Gatsby Good Practical Science project provides a framework for delivering good practical science in schools. Benchmark 9 of the framework is about taking ‘a balanced approach to risk’ and states that students’ experience of practical science should not be restricted by unnecessary risk aversion. In other words, schools should not shy away from practical chemistry. There is plenty of guidance and support available, including model risk assessment.
Teach your students about health, safety and risk throughout the chemistry curriculum, starting on day one in the lab. Begin by asking them to think about and discuss the hazards and risks in everyday life. Look at the How risky is life? resources for ideas on how to help students discuss perceived and actual risk levels. Then move on to some more specific safety issues that we encounter in science. Use the tasks and skills on the CLEAPSS Science safety certificate (part of the freely available student safety sheets) to guide this work. Ensure you have the same lab rules clearly displayed throughout the department – use the CLEAPSS Lab rules poster as a starting point. You’ll find further ideas in this hazards, safety and apparatus article.
Teach your students about health, safety and risk throughout the chemistry curriculum, starting on day one in the lab. Begin by asking them to think about and discuss the hazards and risks in everyday life. Look at the How risky is life? resources for ideas on how to help students discuss perceived and actual risk levels (bit.ly/3Up5yWm). Then move on to some more specific safety issues that we encounter in science. Use the tasks and skills on the CLEAPSS Science safety certificate (part of the freely available student safety sheets) to guide this work. Ensure you have the same lab rules clearly displayed throughout the department – use the CLEAPSS Lab rules poster as a starting point (bit.ly/4ohfcID). You’ll find further ideas in this hazards, safety and apparatus article (rsc.li/40wIEQy).
Progression of ideas and skills
As students progress through school, we expect their conceptual and procedural knowledge and skills to develop (see table below).
| Secondary range | Types of learning areas |
|---|---|
|
Early (approx 11–14) |
Using appropriate techniques, apparatus and materials during lab and field work, paying attention to health and safety Evaluating risks |
|
Mid (approx 14–16) |
Carrying out experiments with due regard for correct manipulation of apparatus, accuracy of measurements and considering health and safety in the lab and field Using specific apparatus to safely use and manipulate solids, liquids and gases, including heating, purification and separation of materials Evaluating risks in practical science and wider societal context, including perception of risk in relation to data and consequences |
|
Late (approx 16–19) |
Safely using a wide range of practical equipment, techniques and materials Identifying hazards, assessing risks and making appropriate safety adjustments when carrying out practical work in the lab or field Considering applications and implications of science and evaluating their associated benefits and risks. |
Practical problems and suggested solutions
Heating
Heating is a key part of practical chemistry as many chemical reactions depend on heating to initiate or sustain the reaction. There are several ways of heating chemicals including the use of Bunsen burners, water baths and electric heaters. Consider the questions and areas to think about in the table below when planning an experiment that involves heating. CLEAPSS Student safety sheets 92–95 provide full guidance for students.
Heating is a key part of practical chemistry as many chemical reactions depend on heating to initiate or sustain the reaction. There are several ways of heating chemicals including the use of Bunsen burners, water baths and electric heaters. Consider the questions and areas to think about in the table below when planning an experiment that involves heating. CLEAPSS Student safety sheets 92–95 provide full guidance for students (bit.ly/4nWUH3s).
| Questions | Things to consider |
|---|---|
|
What personal precautions should you take? |
Safety glasses or goggles? Tie long hair back. Clear working space. Stand or sit? |
|
What are the risks associated with each method of heating: Bunsen burners, water baths, electric heaters? |
Where you are doing the experiment: is there easy access to gas or electricity? If using a Bunsen flame, how do you control the size of the flame so that the clamp doesn’t catch fire? |
|
What temperature is needed for the reaction to work? |
Use a water bath if the temperature is below 100oC. Is it within the temperature range of an electrical heater? |
|
Are any of the reactants or products flammable? |
Avoid using a Bunsen burner near flammable substances, e.g. ethanol, as this reduces the risk of them catching fire. |
|
If the reaction gets too hot will you make any harmful byproducts? |
Control the temperature so the reaction doesn’t get too hot. |
|
How do you avoid the reaction boiling over or shooting out of the reaction vessel? |
Reduce temperature of heater or the size of the Bunsen flame. Use anti-bumping granules. Think about the size of the heating vessel. |
|
Can you do the experiment on a smaller scale or microscale? |
Smaller quantities of chemicals are needed. Spirit burners can be used for heating. |
Handling glassware
Many chemical reactions are carried out in glassware, and we need to handle glass carefully. It is brittle and breaks easily if it is dropped, rolls off the bench or is clamped too tightly. Sudden large temperature changes can cause glass to fracture or shatter (heat shock), for example during a freeze–thaw reaction or suck-back of cold water into a hot reaction vessel. Demonstrate this principle for your students (following a suitable risk assessment) by heating the end of a thin glass rod in a Bunsen flame until it starts to glow, then plunging it into a large trough of cold water.
Glass will flow if it is heated strongly and strongly heating chemicals in cheap glass boiling tubes can cause difficulties. If you are heating, use the more expensive but tougher borosilicate glass (Pyrex) boiling tubes.
To become competent practical chemists, students need to understand these properties of glass and the risks involved. When you introduce or demonstrate experiments, discuss what can go wrong and how to safely use the equipment. Reinforce the safety messages regularly to help ensure your students are well aware of the hazards. Emphasise the reasons behind safety instructions to help students feel more in control and responsible. This awareness will build their practical skills and should encourage them to follow procedures carefully, which will lead to a safer learning environment.
Suck-back
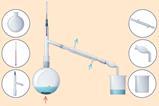
In experiments such as the cracking of hydrocarbons or the thermal decomposition of metal carbonates where carbon dioxide gas is bubbled into limewater, there is always the risk of suck-back (see figures 1 and 2). Suck-back can occur when either no more gas is produced or the heat is removed from the reaction vessel. The pressure of the gas in the heated tube drops, and the external air pressure pushes liquid through the delivery tube towards the hot glassware. Contact between hot glass and room temperature liquids can lead to cracking and shattering of the glass. To minimise the risk of suck-back, lift the delivery tube out of the liquid before stopping the heating. Always use borosilicate glass boiling tubes as these are less likely to shatter in the case of suck-back occurring.

In experiments such as the cracking of hydrocarbons (rsc.li/4eXy22V) or the thermal decomposition of metal carbonates (rsc.li/4kQO9ka) where carbon dioxide gas is bubbled into limewater, there is always the risk of suck-back (see figures 1 and 2). Suck-back can occur when either no more gas is produced or the heat is removed from the reaction vessel. The pressure of the gas in the heated tube drops, and the external air pressure pushes liquid through the delivery tube towards the hot glassware. Contact between hot glass and room temperature liquids can lead to cracking and shattering of the glass. To minimise the risk of suck-back, lift the delivery tube out of the liquid before stopping the heating. Always use borosilicate glass boiling tubes as these are less likely to shatter in the case of suck-back occurring.
Synoptic approach
Preparing copper sulfate crystals in the lab is a straightforward practical activity that most secondary students will do at some point. However, there are a range of safety issues with the standard method often used, including:
- adding solid into hot liquid on a tripod.
- superheating of mixtures causing escape of materials.
- spitting of crystallising solutions.
- copper sulfate decomposing producing toxic sulfur oxide gases.
This video from STEM learning demonstrates some of these effects (start at 20:39). You’ll find plenty of activities and worksheets for developing students’ risk assessment skills relating to this practical in the article, How to teach risk assessment skills.
Use one of the alternative methods provided by CLEAPSS and SSERC, which reduce the risk and provide faster and more reliable outcomes. These methods use a water bath in the first stage, replace the evaporation basin with a conical flask, and use anti-bumping granules. Alternatively consider the microscale preparation of hydrated copper sulfate crystals which uses small quantities of chemicals, replaces the Bunsen burner with a sand tray on an electrical heater and produces good quality crystals without the risks previously discussed.
Preparing copper sulfate crystals (rsc.li/40wqgY1) in the lab is a straightforward practical activity that most secondary students will do at some point. However, there are a range of safety issues with the standard method often used, including:
- adding solid into hot liquid on a tripod.
- superheating of mixtures causing escape of materials.
- spitting of crystallising solutions.
- copper sulfate decomposing producing toxic sulfur oxide gases.
This video from STEM learning demonstrates some of these effects (bit.ly/44WA3YN, start at 20:39). You’ll find plenty of activities and worksheets for developing students’ risk assessment skills relating to this practical in the article, How to teach risk assessment skills (rsc.li/4o0oYhY).
Use one of the alternative methods provided by CLEAPSS and SSERC, which reduce the risk and provide faster and more reliable outcomes. These methods use a water bath in the first stage, replace the evaporation basin with a conical flask, and use anti-bumping granules. Alternatively consider the microscale preparation of hydrated copper sulfate crystals (bit.ly/44Fav3p) which uses small quantities of chemicals, replaces the Bunsen burner with a sand tray on an electrical heater and produces good quality crystals without the risks previously discussed.
Dorothy Warren, an education consultant, originally wrote this article in 2018. Chemistry teacherDavid Paterson updated it in 2025.


Teaching practical science

Boost your confidence and your learners’ outcomes with these articles focused on practical lessons
- 1
 Currently
reading
Currently
reading
Staying safe
- 3
- 4
- 5


























1 Reader's comment