How you can help your students avoid difficulties with organic chemistry
Organic chemistry can appear unattractive to some students at first glance. The terminology it uses can make some see it as a difficult section of chemistry to understand. Fears of the subject area can then be compounded by the complexity of some organic compounds, the requirement to visualise them in 3D and a perceived need to rote learn lots of information. But there are many ways to help students see organic chemistry in all its glory.
Student difficulties

Carbon-based materials are all around us and the diversity of these makes the study of carbon compounds both interesting and confusing. The very word organic has several meanings which may be more familiar to students, referring to the way some food products are produced or to living materials. The terminology we use can be a barrier to learning as students struggle with a whole new ‘language’ of specific terms which, designed to systematise nomenclature, can muddy the waters as we introduce new names for familiar materials. Ethanoic acid for acetic acid and poly(ethene) for polythene, for example. ‘Old’ names persist in industry and in some areas of higher education simply because they are familiar and short and yet the dictates of examinations mean we have to help students to grapple with this whole new language.
Students often fail to connect organic chemistry with other branches of the subject because it is taught in isolation although a more holistic approach in some schemes of work such as Salters’ Advanced Chemistry can avoid this difficulty. Seeing organic chemistry as a series of facts to be learned is often how students perceive the subject and our teaching approach needs to connect ideas to give an integrated structure to students’ thinking. Not least of the difficulties students face is the bewildering complexity of compounds and their 3D structure.
Why is carbon special?
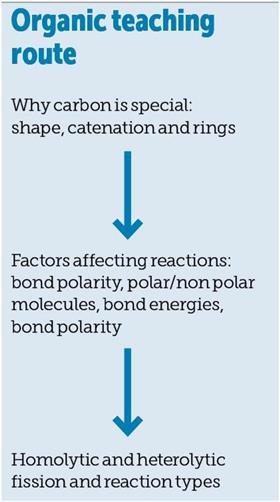
The ability of carbon to form long chains and stable rings of atoms is unique among the elements and this provides a starting point for many teachers. We often introduce simple hydrocarbons and the naming of them to begin our study of organic chemistry. Students can be turned off by rote learning so an approach which includes some mind games such as gridlocks or card sorts can challenge and stimulate them.
Carbon compounds, especially hydrocarbons, are important fuels but the kinetic stability of chains of carbon compounds in this context is counterintuitive. A mixture of hydrocarbon or alcohol vapour and air is thermodynamically unstable, but thankfully kinetically stable at room temperature. Demonstrations such as the ‘whoosh bottle’ can bring this distinction alive and inspire our students at the same time.
The stereochemistry of carbon compounds is crucial to the biological activity of many organic compounds but students often have difficulties visualising the molecules especially if they have only seen 2D drawings of them. There are many types of models available commercially and their use in conjunction with samples of optically active compounds such as carvone can bring this alive and allow the students to appreciate the subtleties of 3D structures. Some schools, and many students, have access to smartphone and tablet apps which also allow the building of 3D images that can be rotated on the screen.
A mechanistic approach
Students find a rote learning approach to organic reactions and mechanisms difficult and unengaging. An alternative method is to use their prior knowledge of electronegativity and electrostatics and consider how these affect interactions between molecules and attacking species. Students may be familiar with this approach if the suggestions made in an earlier article from the CPD series on chemical bonding have been followed.
You can engage students in studying reaction mechanisms by asking them to illustrate the reaction using models: photograph each stage and use a smartphone app to produce an animation of the reaction.
Practical organic chemistry

Organic reactions where preparations require prolonged heating might not give the instant gratification that students find so stimulating in inorganic chemistry. Organic chemicals are also often perceived as hazardous or just plain smelly. Adopting a reduced scale approach to practical organic chemistry can improve manipulative skills, take less time, minimise risks by using small quantities of potentially hazardous or unpleasant chemicals, and generate quiet independent work. Examples of such experiments can be found on Learn Chemistry and from the CLEAPSS advisory service (see guide L215 and these videos).
David Everett is a retired chemistry teacher now working as an independent science education consultant
More CPD
This topic is covered in one of the Royal Society of Chemistry’s Developing Expertise in Teaching courses, designed to support you throughout your teaching career.
Find out more about CPD for Teachers courses on offer.










No comments yet