Medicinal compounds: John Mann takes a look at drugs on the market
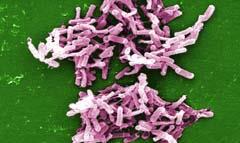
Forget MRSA - Clostridium difficile is the new hospital superbug. With C. difficile infections running at about 55,000 per year in the UK and around 5000 resultant deaths, this gram positive anaerobic bacillus is a cause for concern.
Clostridium difficile is a natural resident in the gut for ca 3 per cent of the adult population, but many patients acquire the bacillus if they spend more than two weeks in hospital especially if they have been treated with broad spectrum antibiotics. These drugs kill most of the natural gut bacterial flora and allow C. difficile to proliferate. The organism is resistant to most antibiotics and produces toxins and 4-hydroxy-toluene (from tyrosine) leading to serious diarrhoea, colitis and, in severe cases in the elderly, to death.
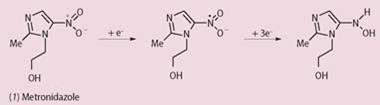
One effective drug against C. difficile is metronidazole (1), a relatively toxic antibacterial agent introduced in 1960 as a treatment for amoebic dysentery (an infection of the intestine by specific amoeba which causes severe diarrhoea). Researchers hypothesise that the drug works by damaging the DNA of the bacillus, and does this after initial reduction of its nitro group via a four-electron process to yield the hydroxylamine. The nitro radical anion and other radical species are possible intermediates of this reduction. A greater understanding of the chemistry of its antibacterial effects may be forthcoming following the unravelling of the biochemistry of C. difficile.
Biochemical breakthrough
A recent paper in Nature (2008, 45, 239) revealed how the organism processes the amino acid l-leucine to 4-methylpentenoate. This amino acid is essential for growth of the bacillus, so this process is a possible target for inhibition by drugs. Jihoe Kim and his colleagues at Philipps Universität, Germany, showed that leucine is first converted into an α-keto acid then reduced to the α-hydroxyacid, at which point the bacillus faces a difficult problem. This type of acid is chemically difficult to dehydrate because the β-hydrogen atom that has to be removed as a proton is not very acidic (pKa around 40). So the bacillus converts the hydroxyacid into a ketyl radical and now the allylic hydrogen atom is more acidic (pKa 14) and can be removed. The overall pathway is shown in the Scheme.
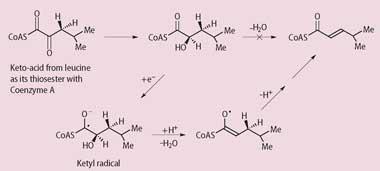
It's too soon to speculate whether the radical intermediates on the metronidazole pathway interact with the ketyl radicalgenerated during leucine reduction, but further investigations of these reductive processes could lead to new drugs for the treatment of C. difficile infections. This is one of the major challenges of the 21st century.






No comments yet