Carbon's noble matchmaker makes Nobel
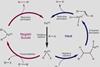
Negishi, Suzuki and Heck were honoured for their pioneering work on palladium catalysis, David Bradley reports
The 2010 Nobel Prize in Chemistrywas awarded jointly to Richard Heck of the University of Delaware, Newark, Ei-ichi Negishi of Purdue University, West Lafayette, Indiana, USA, and Akira Suzuki of Hokkaido University, in Sapporo, Japan, for their pioneering work on palladium-catalysed cross-couplings in organic synthesis. This seemingly innocuous phrase belies the power of a process that allows chemists to join carbon atom to carbon atom and to quickly build complex organic molecules.
Thanks for using Education in Chemistry. You can view one Education in Chemistry article per month as a visitor.

Register for Teach Chemistry for free, unlimited access
Registration is open to all teachers and technicians at secondary schools, colleges and teacher training institutions in the UK and Ireland.
Get all this, plus much more:
- unlimited access to resources, core practical videos and Education in Chemistry articles
- teacher well-being toolkit, personal development resources and online assessments
- applications for funding to support your lessons
Already a Teach Chemistry member? Sign in now.
Not eligible for Teach Chemistry? Sign up for a personal account instead, or you can also access all our resources with Royal Society of Chemistry membership.


