A look at recent chemical science research from Chemistry World
Observing charge distribution in molecules
The distribution of charge across a single molecule has been imaged for the first time by Swiss scientists. It is hoped that this work may eventually lead to electronic devices consisting of organic molecules.
Fabian Mohn and his colleagues at IBM Research-Zurich in Switzerland are well known for their scanning probe microscopy research. So far, they have used atomic force microscopy (AFM) to 'view' all the atoms in a molecule and measure the charge on single atoms, and have also used scanning tunnelling microscopy (STM) to 'see' molecular orbitals. Now, they have used a third mode of the same microscope-Kelvin scanning probe microscopy (KSPM) - to image the charge distribution in a single organic molecule: naphthalocyanine.
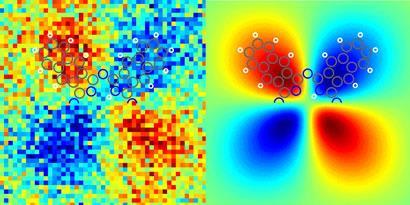
The tip of the microscope is attached to a very small tuning fork, which oscillates at a set speed.The frequency of the oscillations changes slightly when the tip is brought close to a molecule, due to the influence of the forces between the two. In AFM, this so-called detuning is measured at hundreds of points across the molecule, and converted into an image. 'KSPM is very similar, you also measure the force but you measure it as a function of the voltage,' explains Mohn. A voltage is applied between the tip and the conducting naphthalocyanine molecule. 'We look at which voltages the force minimises,' at multiple points across the molecule. This tells them how strong the electric field is at that particular point on the molecule, and allows an image of charge distribution across the molecule to be created.
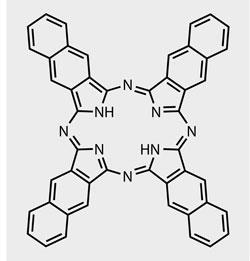
X-shaped naphthalocyanine was chosen as the demonstration molecule because it can switch between two different tautomers when a voltage is applied. The electrons shuffle around the molecule - and arms of the X change from positive to negative and vice versa - when the molecule is switched to its other configuration, Mohn explains. 'This allows us to see how the charge rearranges in the molecule when it switches, and to know immediately that we are not seeing an artefact.'
'They have developed a unique probe to look at the local charge in a molecule,' says Franz Giessibl, an expert in AFM at the University of Regensburg in Germany. He describes the work as a 'very important benchmark' towards the end goal of electronics using organic molecules. Organic electronics would be useful for light-harvesting technologies, electronics for inclusion in clothing and helping to drive down the cost of electronic devices, he says.
The next step for the IBM team is to probe the formation of covalent bonds between two molecules. 'First, you would look at the two molecules separately, and then you would form the bond. After the bond formation you look at how the charge has rearranged in the molecule,' says Mohn.
Nina Notman
Making crisps healthier

An investigation by UK scientists into how salt is released from crisps (known as potato chips in the US) as you eat them could lead to a healthier crisp that tastes just as good.
Ian Fisk and Tian Xing from the University of Nottingham found that a large proportion of the salt in crisps is only released into the mouth 20 seconds after chewing, by which time the crisp may have already been swallowed. Fisk says that this salt burst is underexploited, but it could open doors to salt reduction in snack foods.
Excess salt in the diet has been linked to high blood pressure and cardiovascular disease, so reducing salt in processed foods is a goal for health authorities and food companies alike. 'Our current aim is to develop a series of technologies that accelerates the delivery of salt to the tongue by moving the "burst" from 20 seconds to within the time that you normally chew and swallow,' says Fisk. Scientists could then increase the flavour using less salt.
'Given that people chew and swallow in different ways, it is important to control how they chew and swallow to allow us to understand salt release in the mouth,' says Fisk. He and Xing asked a panel of food testers to chew a crisp a prescribed number of times and hold it in their mouths for 60 seconds. The team monitored the salt level in the mouth throughout the tests by taking tongue swabs and analysing them with flame photometry. After 20 seconds, they detected a peak in salivary salt level. The panellists confirmed that they too sensed a significant increase in salt at around this time.
Salt release is complicated because the salt sits on both the crisp's surface and is embedded in the surface oil, says Fisk. The salt has to be physically separated from the crisp bolus (chewed material), solubilised in saliva and then moved to the salt receptors in the tongue for perception to occur, he explains.
'The work contributes to the fundamental understanding of the phenomena occurring during eating,' says Serafim Bakalis, who investigates how to deliver low salt products without compromising their sensory attributes at the University of Birmingham, UK. 'It will aid in the development of healthier food products without compromising the sensory qualities.'
Fisk is to embark on a research project in March, working with a wide range of large and small food companies in the east midlands in the UK to help them develop effective salt reduction strategies and to solve technical problems surrounding salt reduction.
Elinor Richards
Instant ecstasy detector
An on the spot detector for ecstasy tablets has been made by scientists in Spain. The probe has been designed to detect the active ingredient in ecstasy - MDMA (3,4-methylenedioxymethamphetamine) - even when it is mixed with other common additives, which has been a challenge.
One way to detect ecstasy is a colorimetric test, but the test is not specific for MDMA and is unable to distinguish it from amphetamine and other common phenethylamines. Another method is chromatography, which can distinguish between these compounds but isn't portable. Now, Tomás Torroba and his team from the University of Burgos have made a fluorimetric compound that can be used to identify MDMA. 'The fluorogenic probe may be used as an in situ test for fast detection. It only needs a small sample and can be checked with a portable ultraviolet lamp,' says Torroba.
The probe - a diaryl urea tagged with two fluorescent indicator units - is selective for primary and secondary amines, so it can only reveal that a primary or secondary amine is present. 'It would be difficult to distinguish the difference between amphetamine or MDMA by the naked eye,' says Torroba. 'A mathematical trick is required.'
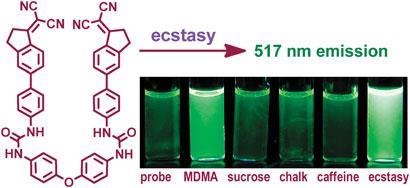
So, the team used a fluorimeter to measure several parameters and ran the resulting numbers through an algorithm. This converted the numbers into a two-dimensional plot in which each component was placed in a different region of the plot. So this standard plot can be used to check for MDMA and other amphetamines in real samples.
To test the method's ability to pick out MDMA when it's mixed with other compounds, the team used it with a tablet consisting of MDMA mixed with sucrose, chalk and caffeine. They compared the fluorescent signal with that of pure MDMA and found that the signals were identical.
'Many tablets of street ecstasy are not pure MDMA and frequently include amphetamines (and may also contain dextromethorphan, caffeine, MDA, ephedrine etc), which may trigger false drug test results,' says Jorge Garrido, who studies amphetamines at the Polytechnic Institute of Porto, Portugal. 'Developing new analytical methods to overcome such a critical limitation is of the utmost importance.'
Elinor Richards
Nanocellulose has paper potential
Patrick Walter/Vancouver, Canada
A nano form of cellulose could soon displace wood pulp in paper, allowing producers to increase the amount of mineral filler they use when they make paper, thanks to work by a Finnish team. This advance would cut the carbon footprint of paper substantially - by 15% or more, the researchers say.

Cellulose is a biopolymer that provides structure in plants. 'Nanocellulose' is a new class of nanomaterial comprising crystalline or fibrous units of cellulose between 5 and 500nm in diameter and potentially hundreds of micrometres in length.
'The basic idea to increase the filler content in paper is a long lasting dream of the papermaker because china clay is typically much less expensive than the cellulose and its carbon footprint is less as it's just a mineral coming from the earth,' says Ali Harlin, a professor of renewable materials at the VTT Technical Research Centre of Finland. By adding 'nano-fibril' cellulose during the production process, wood pulp can be displaced and, because of the superior structural strength of nanocellulose, more filler can be added, cutting production costs by around 3%. Furthermore, it takes about 30% less energy to dry out because much less nanocellulose is needed. Compared with conventional cellulose, the paper is less carbon intensive. 'It's not only cheaper - it's providing better properties, it's less porous, its printing quality is higher and it's not so translucent, so in all respects you get a better paper for less money,' Harlin says.
Paper with an even higher filler content (80%) could be a suitable substrate for printed electronics. Harlin says printed electronics is a rapidly growing field and the nanocellulose paper they've made has a comparable smoothness and stability to conventional plastic substrates like Mylar A.
Theodore Wegner, assistant director of wood, fibre, composites and research at the US Department of Agriculture Forest Service, says that nanocellulose has huge potential. He says it should be possible to cheaply produce nanocellulose in tens of millions of tonnes, unlike conventional nanomaterials that are typically expensive to manufacture. He points out that nanocellulose also has a range of properties that makes it of great interest to materials scientists. The fibres are extremely tough, with a stiffness comparable to that of Kevlar. Furthermore, cellulose nanocrystals display piezoelectric properties comparable to that of quartz and have photonic properties. This means that nanocellulose could find uses in batteries, super capacitors, bioplastics, electronics, as well as a structural material in composites, films or coatings.
Harlin says that paper businesses are taking this technology extremely seriously and that major industrial test facilities are already up and running.
References
- F Mohn et al, Nat. Nanotechnol., 2012, DOI: 10.1038/nnano.2012.20
- T Xing and I D Fisk, Food Funct., 2012, DOI: 10.1039/c2fo10282j
- D Moreno et al, Chem. Commun., 2012, DOI: 10.1039/c2cc17823k






No comments yet