A look at recent chemical science research, contributed by the chemistry world team
Breathing life into medical devices
US scientists have made a device that converts air flow from human breath into electricity. The device could serve as a power source for implantable biomedical devices, removing the need for systems with batteries that need replacing in the operating theatre.
'We've been working on harvesting nano- and micro-scale mechanical energy from human activities for several years, for powering bioimplantable devices and even personal electronics,' explains Xudong Wang, who led the research at the University of Wisconsin-Madison.
Respiration could be an important energy source from the human body, but the air flow rate is low (typically 2m/s) and it fluctuates. Scientists have been able to harvest energy from low speed air flow devices at the centimetre scale and above. But previous devices, such as windmills and inductive wind belts, need wind speeds of over 2m/s to operate. So, a much smaller device is needed to harvest energy from respiration. It also needs to be flexible enough to be placed in the body and tough enough to avoid fatigue failure during long-term use.
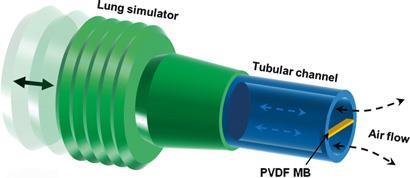
To achieve these goals, Xudong's team designed a micrometre-sized polyvinylidene fluoride (PVDF) belt to harvest the energy. They found that to work under a low speed air flow, the PVDF belt needed to be thin enough to be driven into a resonant oscillation (a deformation that generates an electric current). The major challenge, says Wang, was maintaining the strength of the PVDF while getting it to the correct thickness. To overcome this challenge, the team used an ion etching technique to reduce the belt's thickness.
'Preparing thin PVDF films to harvest energy from weak respiration is an important technology,' says Masao Kaneko, an expert in functional polymers for energy conversion at The Institute of Biophotochemonics, Japan. 'The team should now attempt to drive a real device by the energy accumulated from respiration.'
Wang says his next step is to improve the energy harvesting efficiency and explore more designs for harvesting other types of mechanical energy from the environment or biological systems.
Carl Saxton
Chameleon clothes to detect falling oxygen levels
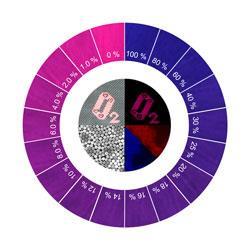
A cloth that changes colour when oxygen levels drop has been developed by scientists in China. The cloth could be used to make clothes that monitor oxygen levels for miners, high altitude adventurers and space explorers.
Xi Chen and colleagues at Xiamen University combined the chameleon cloth with a digital camera to capture real-time images, giving a quick readout of oxygen levels. Locating an oxygen deficiency to prevent danger is very important, says Chen. 'When I saw on television that coal miners were trapped in mines and died because of a lack of oxygen, I felt sad and tried to figure out a simple method to detect oxygen.'
The team made the detector by coating oxygen-sensitive polystyrene microparticles onto cotton thread and embroidering the thread into a piece of cloth. First, the microparticles were loaded with a blue hydrophobic stilbene reference dye and an oxygen-sensitive red dye. 'The biggest challenge was selecting suitable dyes,' says Chen. 'The dyes had to be hydrophobic, photostable, non-toxic (or have low toxicity) and not leak after encapsulation', he adds.
The dyes in the cloth are excited under ultraviolet light and then they emit in the visible range. 'Since the blue reference emission and the red oxygen-dependent emission match the blue and red channels of the chip in digital cameras, respectively, it is possible to analyse the brightness signal recorded in colour images,' explains Chen. The blue dye didn't respond to the oxygen and its intensity remained the same at different concentrations. However, the red dye was very sensitive to oxygen - its red emission decreased with increasing oxygen concentration.
Other colorimetric oxygen sensors have a number of problems: they require special excitation lamps and optical filters, the inhomogeneous dye distribution caused errors, the sensors were rigid and gave a relatively weak luminescence and harmful chemicals were released from the sensing films. 'By fabricating the chameleon cloth, we solved all these problems,' says Chen.
'This is a nice example of taking a synthesis of ideas into the world of "materials science" to make a chemo-responsive material,' says Peter Douglas, an expert in oxygen sensors from Swansea University, UK. 'It is not immediately obvious where it would be used in practice, but now it is available, perhaps real practical applications will follow.'
Elinor Richards
Conjuring up gram quantities of a stabilising anion
For a certain breed of inorganic chemist, weakly coordinating anions are great - they hardly interact with their positive counterparts and so can stabilise unstable cations. But one of the most promising has, until now, been difficult to make. Now, German chemists have succeeded in making a lot more of the anion in one go, but by a rather nasty route.
'It started 15 years ago,' explains Helge Willner, of Bergische University Wuppertal. Willner was working on carbonyl cations that needed weakly interacting anions to stabilise them and one day, during a coffee break it came to him. 'We had the idea to make an anion with a Teflon surface that would be the ideal weakly coordinating anion.'
![B(CF3)4]- 62g of K[B(CF3)4]](https://d1ymz67w5raq8g.cloudfront.net/Pictures/480xAny/5/9/7/112597_fluorination_ic201319h_410_tcm18-209764.jpg)
But making the symmetrical [B(CF3)4]- anion turned out to be difficult. In fact, in the literature Willner and his colleague Eduard Bernhardt found many attempts that only managed to make millimolar amounts. Not really enough for a full investigation of the anion's properties. So, Bernhardt and colleagues set to work trying to solve the problem.
'Ligand substitution doesn't work because boron is too strong a Lewis acid,' says Bernhardt. Instead, Bernhardt and Willner decided to start from a compound where carbon atoms were already attached to the boron - K[B(CN)4]. That hadn't been made before either so Bernhardt made the tetra cyanoborate - which is now often used in ionic liquids - and then set about trying to halogenate it. By reacting the anion with the incredibly toxic interhalogens ClF or ClF3, Bernhardt managed to halogenate each of the cyano groups. That resulted in being able to scale up the process to make tens of grams of K[B(CF3)4].
Nick Norman from the University of Bristol, UK, explains that there's been a lot of theoretical interest in [B(CF3)4]- because it has all the properties that make for good, weakly coordinating anions but that 'the challenge has been to make it in large quantities, which they've now pretty much got round with this new research'. 'There aren't very many groups in the world that could do this sort of chemistry but they have the expertise to do it,' says Norman.
Willner says that now he has enough of the anion to experiment with, he plans to see if it will live up to its promise.
Laura Howes
Using eggshells to remove toxic water pollutants

Scientists in China have developed an absorbent material made from waste eggshell membrane that can remove Cr(VI) from contaminated water.
Chromium is usually found in water systems in two main states: Cr(III) and Cr(VI). But, while Cr(III) is needed for metabolism, Cr(VI) is highly toxic and carcinogenic to living organisms, and unfortunately, this form is highly mobile.
This ability to move quickly in a system means that developing an efficient and cost effective method to remove Cr(VI) from contaminated water is important. Current methods for removal include reduction, ion exchange and absorption. Of these, absorption is popular because it is simple and effective.
Biosorption is a type of absorption that uses a material that is not man-made, usually some kind of waste material. Yuming Huang and Bin Liu from Southwest University, Chongqing, have designed a new biosorbent using eggshells. 'As the by-product of food processing and manufacturing plants, eggshells represent a significant waste because they are traditionally useless after the production of eggs and egg derivatives,' says Huang. 'Using this waste to produce useful biomaterial for removal of Cr(VI) from water may be a good choice and opens up a pathway to using biowaste to treat toxic metals from water.'
Most biosorbents contain amine groups that remove the heavy metals through chelating cationic metal ions and/or absorbing anionic metal species. Eggshell membrane (ESM) contains many surface functional groups, including amines, amides and carboxylic groups. The team modified ESM with polyethyleneimine (PEI), which has known metal chelation properties, and they tested the resulting bioabsorbent for its ability to remove Cr(VI) from water. Huang found that not only did it remove Cr(VI) but it also reduced some of the Cr(VI) to Cr(III), suggesting that PEI-ESM can also detoxify Cr(VI).
'An increasingly diverse range of waste and natural materials have been shown to function as biosorbents for the decontamination of metal bearing waters,' comments Stuart Gibb from the Environmental Research Institute of The University of the Highlands and Islands, Thurso, UK. Gibb says that there are some issues that need to be addressed, but he feels the work shows promise. 'The PEI functionalisation of waste eggshell membrane produces a modified biosorbent of significant novelty and interest,' he adds.
Rebecca Brodie
References
Chengliang Sun et al, Energy Environ. Sci., 2011, (DOI: 10.1039/c1ee02241e)
Xu-dong Wang et al, J. Mater. Chem., 2011, DOI: 10.1039/c1jm14162g
Bin Liu and Yuming Huang, J. Mater. Chem., 2011, DOI: 10.1039/C1JM12329G
Eduard Bernhardt et al, Inorg. Chem., 2011, 50, 10268 (DOI: 10.1021/ic201319h)






No comments yet