In January, five websites for chemistry teachers and their students - covering medicinal, physical and organic chemistry - went live on the Internet. They have been developed by the Royal Society of Chemistry (RSC), working in partnership with industry and educational software companies.
Discover chemistry, the outreach collaborative programme between Pfizer and the RSC has produced two websites.
Masterminding Molecules invites 14-16-year olds to 'crack the code' and win the race to discover a new life-saving medicine. They will need GCSE-level (or equivalent) chemistry and the ability to think logically to complete the mission. As part of a team in competition with another from a rival company, the students have to complete three challenges, which introduce them to the importance of molecular shape and polarity in the design of new medicines. In between each challenge they have to face some basic chemistry questions from a panel of expert scientists.
There are questions on, for example, fuels, the Periodic Table (Groups, structure and bonding), and chemical reactions (acids, alkalis, salts and rates of reaction). The more questions the students get right, the more money they have to spend on testing their lead compounds. They get feedback on how well they are doing at each stage and advice if they seem to be struggling. At the end of the game students are told how well they have done, which includes a report from the experts on their chemical knowledge.
The design studio gives post-16 chemistry students the opportunity to use their knowledge of organic chemistry to discover potential new medicines to treat cancer, HIV, or asthma.
After forming their own company, the students are given an overview of the role of enzymes in disease. They are taken through the relevance of structure and shape to the design of enzyme
inhibitors and how these are linked to potency (ie how tightly the molecule binds to the enzyme) and drug-like properties. The students design molecules by using a drag and drop tool, and can view a three dimensional, molecular model of their design bound in the enzyme active site, before committing the drug to testing.
Following each round, the students' chemistry expertise is tested. The more questions they get right, the more competitive their research becomes since this is linked to a 'dial' which indicates how well the project is going relative to their competitors. When the students identify a possible solution, they submit their molecule for clinical development. At this stage, they are faced with the reality of the challenges and costs associated with the development of new medicines.
Let's get physical
Changing the face of physical chemistry, the collaborative programme between the RSC and Reckitt Benckiser, has developed two new resources to support the teaching of A-level (and equivalent) physical chemistry.
Chemistry in your cupboard presents nine tutorial-style articles on the chemistry that underpins household products produced by Reckitt Benckiser, from pharmaceuticals (Nurofen and Gaviscon) to cleaning materials (Dettol and Cillit Bang).
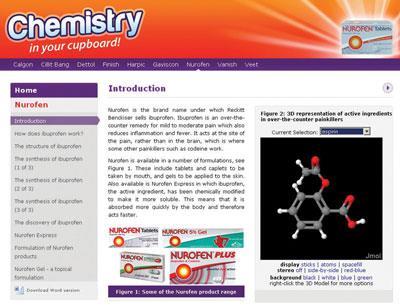
The articles describe the chemistry of these products at a level suitable for post-16 students, covering acids and bases, through polymerisation, to enzyme activity. Links to the curriculum are made clear, and while some tutorials include suggestions for practical work, other have ideas for extension studies and How Science Works.
Questions (and answers) on concepts such as reaction rates or functional group activity are included to promote learning and test understanding. Interactive features include links to rotatable three dimensional, molecular structures and other websites. Each article can be downloaded as a Microsoft Word document, allowing teachers to print and edit the material.
The quantum casino provides a fundamental approach to teaching thermodynamics.4 This starts by considering the chance behaviour of particles and energy, and sees how this leads to the predictable outcomes of chemical reactions.
There are 11 tutorials on, for example, the direction of chemical reactions, entropy, and Gibbs free energy, These are accompanied by simulations (of energy distributions, approach to equilibrium etc), video clips (eg explaining the direction of chemical reactions and entropy within different states of matter), statistical models and graphical analyses and predictions. The tutorials form a narrative, which could be used as a teaching sequence, and the simulations and video clips can be accessed from this narrative, or independently. In the latter case, they are available as a 'tool kit' that can be used as teacher demonstrations or as independent student investigations.
Organic, naturally
Together with ShirePharmaceuticals, the RSC has developed a post-16 resource focusing on organic chemistry.5
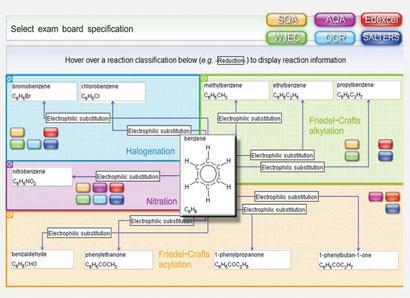
Synthesis explorer curriculum-relevant resource, which allows students to choose from hundreds of starting compounds, react them and view details of the reaction conditions and reagents. The built in jmol viewer also allows users to view three dimensional versions of the compounds, which gives a clearer understanding of chemical structure. Students can examine physical, structural and spectral data for each compound. The spectral data are useful to illustrate reactions that involve changes in functional groups and reflect the increasing requirements for understanding spectroscopic data in current post-16 specifications.






No comments yet