Caffeine-fuelled fix for runaway eye treatment
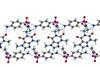
Crystal designed to have stronger hydrogen bonds
Sulfacetamide (SACT) is often lost on blinking and in tears when applied as a treatment for conjunctivitis and other ocular ailments. This leads to the inconvenience and complications of applying larger and more frequent doses of SACT.
Various schemes have been investigated, including trapping SACT in bioadhesive microspheres, to slow drug release and prevent its washout. But these either limited the drug’s bioavailability or weren’t suitable to market.
To solve the problem, Ashwini Nangia and colleagues at the University of Hyderabad in India, looked to cocrystals.
This article provides a link to coverage by Chemistry World
Thanks for using Education in Chemistry. You can view one Education in Chemistry article per month as a visitor.

Register for Teach Chemistry for free, unlimited access
Registration is open to all teachers and technicians at secondary schools, colleges and teacher training institutions in the UK and Ireland.
Get all this, plus much more:
- unlimited access to resources, core practical videos and Education in Chemistry articles
- teacher well-being toolkit, personal development resources and online assessments
- applications for funding to support your lessons
Already a Teach Chemistry member? Sign in now.
Not eligible for Teach Chemistry? Sign up for a personal account instead, or you can also access all our resources with Royal Society of Chemistry membership.


