The new device that combines water desalination with electrolysis to produce green hydrogen from seawater
-

Download this
Use this story and the accompanying summary slide to add new context when teaching about electrolysis with your 14–16 learners.
Download the story as MS Word or PDF and the summary slide as MS PowerPoint or PDF.
Scientists in China have developed and tested a dual desalination–electrolysis device that produces hydrogen gas from saltwater. Their integrated system is one of the first practical and scalable methods for generating hydrogen fuel from non-potable water.
Hydrogen is widely touted as an eco-friendly alternative to fossil fuels since it doesn’t produce polluting gases when burned. Unfortunately, most hydrogen fuel used today is still produced by steam reforming natural gas (methane).
Hydrogen fuel can also be produced from splitting water, using renewable electricity, and there is enormous interest in scaling up this less harmful approach to hydrogen production. As saltwater is so abundant on Earth, to have an alternative to drinking water would be an added bonus.
Saltwater split
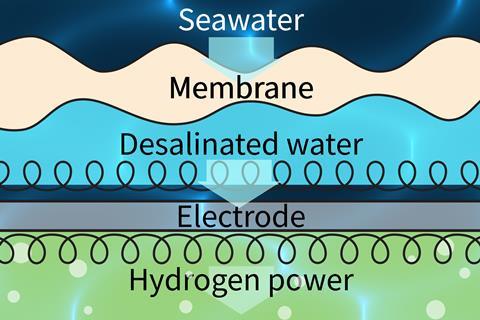
Electrolysis is the process splitting water into hydrogen and oxygen using electricity. Although it is a well-studied process, it is currently difficult to achieve affordably, especially on a large scale. The impurities in saltwater also pose an additional challenge. Chloride ions from the saltwater are turned to chlorine gas at the anodes of the electrochemical cells. This extremely corrosive gas degrades the electrodes, rapidly inactivating the cells.
‘Desalinating seawater before electrolysis can eliminate [these] problems,’ says Zongping Shao, at Nanjing Tech University in China, who led this research. Currently, the energy cost of removing salt from the water outweighs the value of hydrogen generated by water splitting.
Low-power approach
Zongping and his colleagues use reverse osmosis to generate pure water from seawater inside the same device that houses the electrochemical cell. A membrane in the device separates the seawater from the electrolysis components.
The membrane contains tiny pores that water, salt and other impurities cannot pass through. It’s possible, however, for single molecules of water vapour (produced by evaporation) to squeeze through. Waterproof coats have similar ‘breathable’ membranes that allow sweat to escape while protecting the wearer against the elements. Importantly, the reverse osmosis process in the device doesn’t require any energy input.
Once the water has reached the electrolysis cell on the other side of the membrane, it is split into hydrogen and oxygen.
Out in the field
The scientists have already demonstrated their combined desalination–electrolysis device under real-life conditions. They installed a demonstration device in the ocean at Shenzhen Bay, China, and monitored its performance for 133 days. During this time, they collected over a million litres of pure hydrogen produced by the device without any signs of corrosion in the electrochemical cell.
‘It’s a nice demonstration of the technical feasibility of carrying out direct seawater electrolysis for prolonged periods without any apparent loss in activity,’ explains Alexander Cowan, a sustainable fuels researcher at the University of Liverpool, UK, who was not involved in this project.
Put this in context
Explore the role of an environmental process specialist working in the steel industry to improve processes and reduce their impact on the environment.
This article is adapted from Victoria Atkinson’s in Chemistry World.
Nina Notman
Reference
H Xie et al, Nature, 2022, 612, 673–678 (DOI: 10.1038/s41586-022-05379-5)
Download this
Download this article and a one-slide summary with questions to use with your 14–16 students when teaching electrolysis: rsc.li/3XJN2Iy
Downloads
EiC summary slide Electrolysing seawater
Presentation | PDF, Size 0.29 mbEiC summary slide Electrolysing seawater
Presentation | PowerPoint, Size 1.67 mbEiC science research story Electrolysing seawater
Handout | PDF, Size 0.16 mbEiC science research story Electrolysing seawater
Handout | Word, Size 0.5 mb














No comments yet