Gold nanoparticles confirmed as the reason for fulminating gold’s vibrantly coloured smoke
-

Download this
Use this story and the accompanying summary slide for a real-world context when studying nanoparticles with your 14–16 learners.
Download the story as MS Word or PDF and the summary slide as MS PowerPoint or PDF.
The mystery behind why the first reported high explosive, fulminating gold, produces a purple smoke when it detonates has been solved, finally resolving a 400 year-old alchemical puzzle.
Fulminating gold is a mixture of a number of compounds, with gold(III) compounds complexed with ammonia providing most of its explosive punch. Its name comes from the Latin word fulmen, which means lightning.
Fulminating gold was first mentioned by alchemists in the 16th century. When German alchemist Sebald Schwaertzer described its synthesis in 1585, he noted the unusual purple smoke given off when it was detonated. Major scientific figures of the 17th and 18th century – including Robert Hooke, Carl Wilhelm Scheele and Antoine Lavoisier – went on to study it. The material even temporarily blinded Jöns Jakob Berzelius when he dropped a beaker containing a sample in 1809, resulting in glass shards in his eye and permanent, purple-coloured scars on his hand.
However, while the fulminating gold recipe has been understood for centuries, one question has lingered: why does its detonation produce purple smoke?
A smoke trap
Now, Simon Hall’s team at the University of Bristol has confirmed the answer. The most common explanation for the purple smoke is that it contains gold nanoparticles, which look purple because of the way they interact with light. However, the presence of gold nanoparticles in smoke from fulminating gold had never been confirmed experimentally.
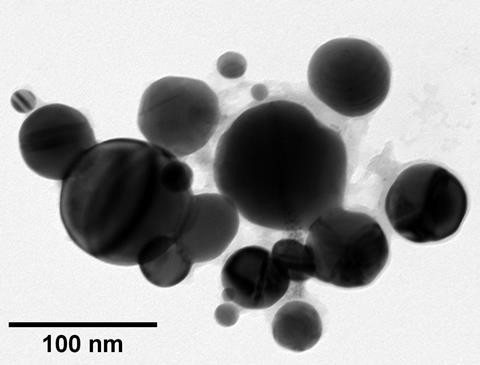
The team discovered the smoke from fulminating gold contains spherical gold nanoparticles ranging in size from 30 nm to 300 nm, confirming the theory that the nanoparticles were responsible for the mysterious colour of the smoke. The particles’ presence also explains other mysteries related to fulminating gold, including why Berzelius’ scars were purple, and how it was used by alchemists to coat objects in a purple patina.
‘The work confirms the long-held assumption that the purple fumes of fulminating gold contain elemental gold in the form of nanoparticles,’ says Curt Wentrup, an organic chemist at the University of Queensland, who has researched the topic in the past.
Simon and his team now plan to use the same experimental approach to study the smoke produced by other metal fulminates such as platinum, silver, lead and mercury.
This article is adapted from Kit Chapman’s in Chemistry World.
Nina Notman
Reference
J M Uszko et al, arXiv, 2023, DOI: 10.48550/arXiv.2310.15125
Download this
Summary slide with questions and the article for context when teaching 14–16 classes on the nanoparticles: rsc.li/46SoIbe
The team discovered the smoke from fulminating gold contains spherical gold nanoparticles
Downloads
EiC summary slide explosive gold
Presentation | PDF, Size 0.24 mbEiC summary slide explosive gold
Presentation | PowerPoint, Size 0.65 mbNanoparticles unlock the secrets to old alchemical puzzle student sheet
Handout | PDF, Size 0.18 mbNanoparticles unlock the secrets to old alchemical puzzle student sheet
Handout | Word, Size 0.54 mb














No comments yet