New system that uses the Earth’s seawater sink has advantages over existing systems that capture carbon dioxide gas from air
-

Download this
Use this story and the accompanying summary slide to add new context when teaching atmosphere and reducing carbon footprint to your 14–16 learners.
Download the story as MS Word or PDF and the summary slide as MS PowerPoint or PDF
Capturing carbon dioxide (CO2) from seawater to address climate change could be more efficient and cheaper than existing systems that capture it from ambient air, say engineers in the US. Oceans and other surface waters act as large carbon sinks and have absorbed 30% to 40% of CO2 emissions from human activities since the industrial revolution began.
Ocean capture
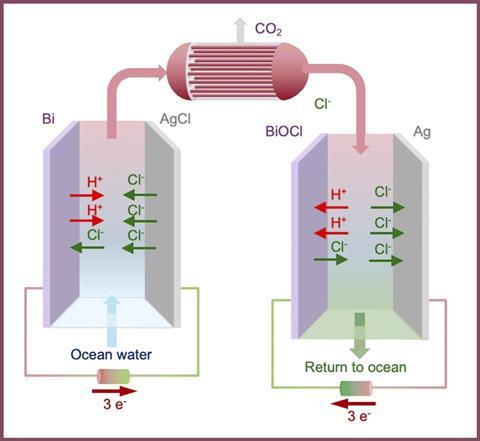
Carbon dioxide reacts with seawater to form carbonic acid, which can then dissociate to form bicarbonate (and other) ions. The engineers at the Massachusetts Institute of Technology (MIT) have developed an electrochemical system that converts the bicarbonate ions back into CO2 gas that can then be collected.
The MIT team’s ocean capture system uses a bismuth electrode that reacts with the chloride ions naturally present in salt water, producing a bismuth compound and protons. These protons increase the acidity of the water, which causes the bicarbonates to break down and reform CO2. The water is then returned to its original pH before it is released back into the ocean.
Making space
Releasing CO2 from ocean water in this way enables it to capture more CO2 from the air. ‘Anthropogenic CO2 distributes between the ambient air and the ocean water, so removing it from the oceans means there is a drive for more to be absorbed [by the water] and thereby be removed from the air,’ says Alan Hatton, one of the study’s co-authors. ‘The net result of what we do is to remove CO2 from the environment as a whole,’ he adds.
The team’s ocean capture system could be more efficient than air capture systems because the concentration of dissolved CO2 in seawater is more than 100 times greater than it is in air, Alan notes. A preliminary cost analysis suggests that this technology would be economically feasible, with costs ranging from $50–$100 (£42–£83) per tonne of CO2. For comparison, one air capture system was calculated to cost $600 per tonne of CO2 in 2020.
Finding a purpose
Once the CO2 is extracted from the seawater, the question becomes what to do with it. Alan believes there are two possibilities: CO2 can be turned into fuels, chemicals and materials, or it can be permanently sequestered (stored) in rock formations underground. Both these solutions are already in use for CO2 captured from air.
The MIT engineers are currently patenting their ocean capture system. At first, the idea is to couple the technology with existing infrastructure that already processes seawater, such as desalination plants that remove salt to create drinking water. The team’s end goal is for its system to be deployed as free-standing ocean capture plants around the world.
This article is adapted from Rebecca Trager’s in Chemistry World.
Dissolved CO2 in seawater is more than 100 times greater than it is in air
Oceans have absorbed 30% to 40% of CO2 emissions from human activities
Nina Notman
Reference
S Kim et al, Energy Environ. Sci., 2023, DOI: 10.1039/d2ee03804h
Download this
Summary slide with questions and the article for context when teaching your 14–16 classes on atmosphere and reducing carbon footprint: rsc.li/3Z10mYL
Downloads
EiC summary slide Carbon footprint reduction
Presentation | PDF, Size 0.3 mbEiC summary slide Carbon footprint reduction
Presentation | PowerPoint, Size 2.5 mbEiC science research story Carbon footprint reduction
Handout | PDF, Size 0.29 mbEiC science research story Carbon footprint reduction
Handout | Word, Size 0.71 mb














No comments yet