Simon Cotton, teacher at Uppingham School, takes a look at those compounds that find themselves in the news or relate to our everyday lives.
In this issue: bisphenol A (BPA)
Why is this chemical important?
Bisphenol A or 2-2-bis(4-hydroxyphenyl) propane (1) is the raw material for the manufacture of polycarbonate (2), and other polymers. Polycarbonate is a strong material transparent to visible light, though opaque to ultraviolet. Polycarbonate has many applications and it is widely used to make reusable plastic baby bottles and food containers, eye lenses, even strengthened glass.
How is bisphenol A made?
It's made by a condensation reaction between phenol and propanone (acetone), using an acid catalyst such as hydrochloric acid. Annual production of BPA is two-three million tonnes.
And polycarbonate?
Another condensation reaction, this time involving bisphenol A and phosgene, using a base such as sodium hydroxide to mop up the HCl.
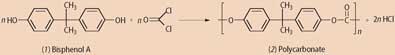
Why has BPA been in the news?

There have been concerns that BPA can leak out of polycarbonate products, in particular baby bottles, when the material is heated. Bisphenol A was first made in 1891 by Russian chemist Aleksandr Dianin. By the end of the 1930s E. C. Dodds and Wilfred Lawson working at Middlesex Hopsital had shown that BPA has similar activity to the sex hormone oestrogen produced in the body. By imitating oestrogen, BPA disrupts chemical signalling in the endocrine system.
So does it leak out?
Results from tests on a sample of over 2000 people published by the US Centers for Disease Control and Prevention in October 2007 showed that 93 per cent of the sample had detectable levels of BPA in their urine, though in amounts far below recommended daily intake levels. The question is whether the amount released (at the ppb level) is high enough to be dangerous.
Well, is it?
Opinion appears to be divided. In March 2009, six US manufacturers of baby bottles decided to remove BPA from their products in response to consumer demand. In April 2008 the Canadian public health authority labelled BPA a hazardous substance, which led to a ban on the import and sale of bottles containing BPA. In September 2008 research by a group led by Dr Iain Lang of the Peninsula Medical School, Exeter, found some correlations between adults with high levels of BPA in their body and the occurrence of heart disease and diabetes.
However, a US National Toxicology Program report on exposure to BPA published in September 2008 said 'there is no direct evidence that exposure of people to bisphenol A adversely affects reproduction or development'. And according to the US Food and Drug Administration (FDA), the consensus among regulatory bodies around the world is that 'current levels of exposure to BPA through food packaging do not pose an immediate health risk to the general population, including infants and babies'.






No comments yet