I find it hard to choose when students ask me for my favourite element, but phosphorus ranks quite highly. Like carbon and sulfur, it displays a range of allotropes with interesting structures and properties to discuss. One of the most iconic phosphorus demonstrations involves igniting white phosphorus in a large round bottomed flask filled with oxygen to produce a magical glowing ‘sun’.
Many think the experiment can only be performed with white phosphorus, which is hard to acquire and difficult to handle safely. But this isn’t the case – exactly the same astonishing effect can be produced with red phosphorus.

Kit
- Red phosphorus (about 0.4 g)
- Clean deflagrating spoon
- 1 dm3 borosilicate round bottomed flask filled with oxygen – preferably thick-walled. I use a 10 dm3 flask but the demonstration works just as well in smaller flasks.
- Means to secure the flask (clamp and retort stand or cork ring)
- Delivery tube
- 1 dm3 conical flask for use as a fume trap
- Universal indicator solution
- Safety screens if in open lab
Preparation
Work in a well-ventilated room if working on a small scale (using safety screens to protect audience and demonstrator) or preferably in a fume cupboard. Fill the round bottomed flask with oxygen and clamp it into position. Add water and universal indicator solution to the conical flask and submerge the end of the delivery tube such that any expelled gases bubble through the water.
In front of the audience
Both the deflagrating spoon and delivery tube will need to go through the bung. The spoon has too small a diameter for a standard 2-hole bung so a special bung will need to be bored.
Place the phosphorus into the bowl of the deflagrating spoon (about 0.4 g) and lower it into the round bottomed flask. Hold the end of a glass rod in a roaring Bunsen flame for about 30 seconds and then touch the hot end of the rod against the phosphorus, which will light.
As you seal the flask, any acidic gases will dissolve and react with the water in the conical flask. Ensure you remove the end of the delivery tube as soon as bubbling stops in order to prevent suck-back.
Hypnotic white tendrils of smoke fill the flask, which glows like a miniature sun. Once the reaction is complete, ensure the flask is cool before adding the water and indicator from the conical flask to the round bottomed flask to help dissolve the P4O10 mist. The solution will turn red as phosphoric acid is formed.
Teaching goal
This demonstration is ideal for use with lessons on periodicity. Lower school students may need to appreciate that the oxides of non-metals form acids and that these play a role in acid deposition. Older students are often required to know the properties of the period 3 elements and the reactions of their oxides with water.
The reaction we see is
P4 + 5O2 → P4O10
Followed by reaction with water to produce phosphoric acid:
P4O10 + 6H2O → 4H3PO4
Despite having a similar formula to sulfuric acid, phosphoric acid is significantly weaker and makes for an interesting discussion about the limits of the assumptions made in acid-base calculations. It clearly cannot be assumed to completely dissociate, but with the first pKa around 2, are calculations of pH to three significant figures possible when using the standard weak acid assumptions?
A calculation based on traditional weak acid assumptions returns a pH of 1.06 for a 1 mol dm-3 solution. But accounting for the drop in HA by solving the quadratic equation (below) of the first Ka expression (before adding the H+ from the other terms using a traditional approach) returns a pH of 1.08.
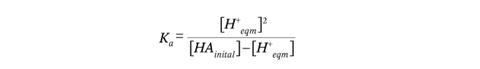
The structures of the allotropes of phosphorus present a number of interesting properties that make it an ideal element to discuss with older students. Before showing my students phosphorus, I ask them to draw its Lewis structure and use this to predict its properties. Inevitably, they draw a diatomic structure analogous to nitrogen.
This is a chance to discuss the effect of atomic radius on the strength of the π-bond and the consequences for P, S and SiO2 (when compared with CO2).
From a simple bond enthalpy perspective, the formation of double and triple bonds for the smaller two elements is favourable. But for the larger two, the increased atomic radius and corresponding reduction in p-orbital overlap favours the formation of multiple single bonds. This is why oxygen and nitrogen are diatomic while sulfur exists as rings and phosphorus exists in its various allotropes.
| Element | X–X single bond strength (kJ mol-1) | 2 x X–X single bond strength (kJ mol-1) | X–X double bond strength (kJ mol-1) | 3 x X–X single bond strength (kJ mol-1) | X–X triple bond strength (kJ mol-1) |
| N |
161 |
322 |
456 |
482 |
946 |
| O |
147 |
293 |
498 |
--- |
--- |
| P |
209 |
419 |
351 |
628 |
490 |
| S |
226 |
452 |
425 |
--- |
--- |
Safety
- Wear eye protection.
- Do not attempt to dry red phosphorus in an oven if it looks damp.
- You may be able to pat it dry enough with a paper towel.
Disposal
The acidic solution produced is dilute enough to be poured down the sink. Any excess red phosphorous on the spoon will need to be burned off in a fume cupboard.




















No comments yet