Demonstrations designed to capture the student's imagination
Demonstrations to capture the student's imagination by Adrian Guy of Blundell's School. In this issue: Floating Gas Bubbles

Several years ago, when I also taught physics, we used a demonstration to compare densities of liquids and solids called the 'knickerbocker glory' of densities. This was demonstrated in a tall gas jar and involved paraffin floating on water. Different solids were then added: ice floated on the water but below the paraffin (between the paraffin/water boundary), polystyrene floated on the very top (on the paraffin), whilst metal objects sank to the very bottom.
Then, many years later I was watching the popular US TV series MythBusters where they managed to float a paper boat on carbon disulfide gas. This gave me the idea of floating air bubbles on carbon dioxide.
This demonstration is enjoyed by all ages, but is particularly useful for Year 8 students when studying the properties of gases. It helps to reinforce the idea of density, which can be related to methods of collecting gases (upwards displacement of air etc), and to enforce the idea that gases, despite many being colourless, still have substance.
Kit
- 250 cm3 beaker
- small marble chips (approx 4-6 mm)
- 500 cm3 of 2 M HCl
- large acid resistant container such a glass trough or large plastic box
- soap bubbles - produced from an inexpensive kids toy or similar
Procedure
Spread 250 cm3 of marble chips, or enough to cover the surface of your container, then add 500 cm3 of 2 M HCl, or enough to cover the marble.
Avoiding draughts, allowing the reaction between the marble and acid to continue until completion, ie when the effervescence stops.
Standing back, so that the draught from blowing bubbles does not disturb and therefore mix the carbon dioxide with surrounding air, blow bubbles so they fall on top of the carbon dioxide where they will float for a substantial period.
Special tips
- Wait for the reaction between the acid and marble to subside before blowing bubbles, as the fine spray of acid ejected with the bursting bubbles pops the bubbles, or shortens their life expectancy.
- Keep all drafts and pupil movements to a minimum.
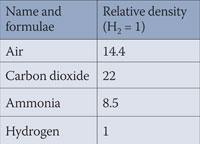
Table 1: Density of some gases1
Theory
Whilst the ideal behaviour of gases predicts that the molar volume of any gas is 24.5 dm3 at 298 K assuming perfect gaseous behaviour, their densities will differ depending on the relative molecular mass Mr. Carbon dioxide with a Mr of 44 g mol-1 should therefore be 22 times the density of hydrogen with an Mr of 2 g mol-1. So, providing the bubbles of exhaled air including the liquid bubble combined have a lower density than the equivalent volume of carbon dioxide, the bubble will float.
A more scientific method could be used to calculate this, involving estimating the size of a bubble (its volume), and estimating the mass by landing a bubble of exhaled air on a sensitive scale. Calculations could be made to work out the volume of carbon dioxide that would need to be displaced for weight to equal upthrust.
Further work
Given the information in Table 1 or using Mr or density data for various gases, devise combinations of gases that will float on one another. Better still, try this for a combination of gases where, for example, bubbles of ammonia will rise into a container of hydrogen (less dense) and appear suspended.
What else will float on carbon dioxide - will a normal party balloon of air still float?
Safety
- Chlorine and hydrogen can explode spontaneously in sunlight and various other combinations of oxygen and combustible gases can form explosive mixtures.
- 2 M Hydrochloric acid is an irritant to the eyes and skin - eye protection and gloves should be used.
- Do not exceed the quantities in this experiment, especially in small, confined spaces where the level of carbon dioxide could be an issue.
References
- L Davies et al, Investigating Chemistry, p105. Heinemann Education Books Ltd, 1973









No comments yet