Get started
Watch a demonstration of this experiment and download the technician notes from the Education in Chemistry website: rsc.li/3TaTHKv
Although chemistry textbooks typically depict substances as consisting of particles that are spherical in shape, the reality is somewhat more complicated. From proteins to plastics, we can better explain the properties of many materials that we encounter every day in terms of particles resembling chains of beads. You can demonstrate this idea with a liquid that you can cut and siphons out of a beaker.
Use the experiment when teaching polymers and their properties to learners aged 11–16. You could also use it when teaching hydrogen bonding at post-16.
Kit
- 4 g high molecular weight poly(ethylene glycol), also known as poly(ethane-1,2-diol) and polyox – see tips
- 25 cm3 propan-2-ol
- Stirring rod
- 100 cm3 beaker
- 1 L beaker
- 600 cm3 beakers × 2
- Food dye
- Scissors
- Clamp and stand
Preparation
Add 3–4 g of poly(ethylene glycol), PEG, to a 100 cm3 beaker. Add 25 cm3 of propan-2-ol and stir rapidly to disperse the powder. Add 10 drops of a food dye to the mixture at this stage to help you see the effect more clearly.
Add approximately 400 cm3 of water to a 1 L beaker and tip the dispersed mixture as quickly as possible into the water. Continue mixing with a stirring rod until the solution thickens and begins to climb the rod. Leave it for a few hours to fully hydrate before use.
You can keep the solution in a sealed bottle for several months. If the viscosity drops on standing, you can revive it by sprinkling some extra PEG powder onto the surface of the solution, shaking and leaving it for 24 hours to dissolve.
Health, safety and disposal
- Read our standard health and safety guidance and carry out a risk assessment before running a live demonstration.
- Wear eye protection.
- Propan-2-ol is a highly flammable liquid and the vapour may cause drowsiness/dizziness. CLEAPSS members should consult HC084A.
- Poly(ethylene glycol) solid is classed as low hazard. The solution can be rather slippery and has some unusual/unexpected properties so you should handle it with care – including clearing up any spills. Hazard classification may vary depending on supplier. To dispose of, dilute the solution and pour it down a foul-water drain.
Health, safety and disposal
- Read our standard health and safety guidance (rsc.li/3TZoxYh) and carry out a risk assessment before running a live demonstration.
- Wear eye protection.
- Propan-2-ol is a highly flammable liquid and the vapour may cause drowsiness/dizziness. CLEAPSS members should consult HC084A: bit.ly/4axUs7q
- Poly(ethylene glycol) solid is classed as low hazard. The solution can be rather slippery and has some unusual/unexpected properties so you should handle it with care – including clearing up any spills. Hazard classification may vary depending on supplier. Dilute the solution and pour it down a foul-water drain.
In front of the class
Use your prepared PEG solution to demonstrate the polymer’s unusual properties.
Self-siphoning liquid
Fill one of the 600 cm3 beakers with your PEG solution. Place the clamp stand in your demonstrating space and put the second 600 cm3 beaker (dry and empty) next to the base of the clamp stand. Clamp the PEG solution beaker with its spout above the empty beaker Tip the upper beaker gently until the liquid starts to pour out of the spout into the second beaker. Keep the angle of the upper beaker constant and carefully elevate it by moving the clamp’s height to lengthen the stream.
Even as the level of liquid in the upper beaker drops beneath the level of its spout, the liquid will continue pouring out until it is nearly empty.
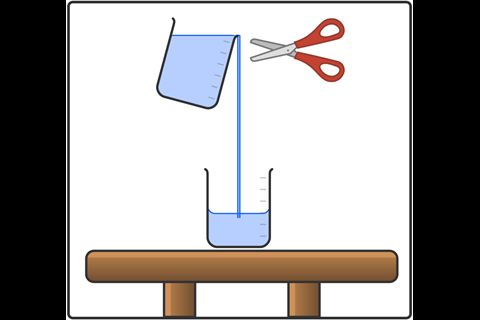
Cuttable liquid
Demonstrate the unusual cohesive forces in the liquid by cutting the solution. To do this, place an empty 600 cm3 beaker in your demonstrating space, hold the beaker with your PEG solution above it and pour the liquid into the empty beaker. Pour over the edge, not the spout, of the beaker. Once you have established a good flow of liquid, return the upper beaker nearly to vertical (less than 30° of tilt). Using sharp scissors in your other hand, cut through the liquid approximately 1 cm from the rim of the upper beaker and keep the blade moving as you cut for best results. You will see the part of the liquid just above the cut spring back into the upper beaker.
Tips
- Educational suppliers sell the colourless solid required for this demonstration as polyox, but you can also buy it from chemical suppliers as PEG, poly(ethylene glycol), or PEO, poly(ethylene oxide).
- This demo requires chains of approximately 90,000 repeating units that weigh 4,000,000 g mol–1. Suppliers may label this PEG–90M, where M is thousands-of-monomers, or WSR 301.
- Do not use products that contain shorter chains labelled simply with a number, such as PEG 400. Here the number indicates the molecular weight. Solutions made from these shorter chains will not produce the effect described here.
Teaching goal
Poly(ethylene glycol) is used widely in drug delivery, wound dressings, artefact conservation, creams, pastes, cosmetics and more recently mRNA vaccines. The effects seen here result from the solution’s viscoelastic properties – it acts a bit like a liquid and a bit like a solid. The material is viscous, a property of liquids that you can experience in the resistance when you try to stir it. It is also elastic (resists deformation), a property more associated with solids.
Tips
- Educational suppliers sell the colourless solid required for this demonstration as polyox, but you can also buy it from chemical suppliers as PEG, poly(ethylene glycol) or PEO, poly(ethylene oxide).
- This demo requires chains of around 90,000 repeating units that weigh approximately 4,000,000 g mol–1. Suppliers may label this PEG–90M, where M is thousands-of-monomers, or WSR 301.
- Do not use products that contain shorter chains labelled simply with a number, such as PEG 400. Here the number indicates the molecular weight. Solutions made from these shorter chains will not produce the effect described here.
The poly(ethylene glycol) particles are more like long chains than small sphere-like molecules. Chemists make the chains by condensation addition polymerisation of ethane-1,2-diol (better known as ethylene glycol) as shown.
n HO–CH2 –CH2 –OH → HO–(CH2 –CH2 –O)n –H + n H2 O
Every third atom in the chain is an oxygen, which can accept hydrogen bonds from water, and this helps the two substances to mix.
These chains become entangled as you apply a force – like the weight of the liquid pulling down on the stream of fluid – causing them to display more elastic properties. You can model this with a pile of rope or Christmas bead decorations: if you pull quickly, the material will knot up (modelling elastic behaviour), but if you pull slowly, the chains will disentangle and separate (modelling viscous behaviour).
More resources
- Have fun with the borax/PVA slime experiment to create seaweed spheres and demonstrate the cross-linking in sodium alginate to create gels.
- Investigate intermolecular forces and the properties of polymers with the perturbed polymers demonstration when teaching polymers’ sensitivity to particular solvents to 11–14 and 14–16 learners.
- Use this resource on addition polymerisation to help your 14–16 students develop how to name polymers and draw their structures. Plus, display the poster in your classroom.
- Explore Newtonian and non-Newtonian fluids with the custard and quicksand activities in our Outreach resources hub.
Declan Fleming
Downloads
Self-pouring polymer technician notes
Handout | PDF, Size 0.27 mbSelf-pouring polymer technician notes
Editable handout | Word, Size 0.52 mb
























No comments yet