Arthur C Clarke’s famous third law: ‘any sufficiently advanced technology is indistinguishable from magic’ can also be applied to chemistry. There’s something about an oscillating reaction that really makes a student’s jaw drop and ask questions. While there are a variety of such reactions out there, perhaps the most iconic is the Briggs–Rauscher reaction. The rotating orange–black–colourless changes are incredibly reliable and the solutions, once made up, can tolerate a wide spectrum of concentrations. This makes this a reliable and satisfying demonstration that can be used to stimulate wonder or demonstrate aspects of reaction mechanisms and autocatalytic behaviour.
Kit
The following quantities are suggested for one demonstration – larger quantities can be made for repeats.
- 20 cm3 of 10 vol hydrogen peroxide solution
- 1.2 g potassium iodate(V) (oxidising, irritating, harmful if swallowed)
- 20 cm3 of sulfuric acid (0.1 M)
- 0.4 g of malonic (propanedioic) acid (irritating, harmful if swallowed)
- 0.09 g manganese sulfate(VI) monohydrate (may cause damage to organs through repeated/prolonged exposure, toxic to aquatic life)
- magnetic stirrer and follower
- 100 cm3 conical flask or beaker
- 1 cm3 of fresh 1% starch solution
Preparation
Wear eye protection and avoid raising dust when weighing out the solids. Label the hydrogen peroxide solution ‘A’. Dissolve 1.2 g potassium iodate(V) in 20 cm3 of sulfuric acid (0.1 M) and label this solution ‘B’. Label the starch solution ‘C’. Dissolve 0.4 g malonic acid and 0.09 g manganese sulfate(VI) monohydrate in 20 cm3 of deionised water – label this solution ‘D’.

In front of the class
Add the solutions to a beaker or conical flask placed on a magnetic stirrer in alphabetical order. After a short induction period, the colourless solution will turn orange, then black, and return to colourless before repeating for approximately five minutes – finally settling in the black form.
Teaching goal
The overall reactions competing with one another have been suggested to be:
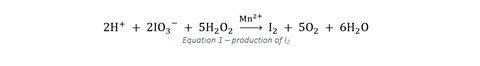
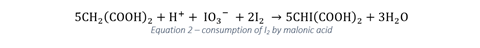
Initially the concentration of I2 and I–, both of which are required for the production of the classic blue–black starch complex, are low. Until the concentration of I– reaches 10–4 M, the complex cannot form (which explains the orange → black transition) and it is the swinging of the I– concentration that is responsible for the most dramatic changes in the oscillator. The free iodine is consumed by malonic acid until the concentration of I2 drops below that required for the complex to form, leading to the switch back to colourless – at which point reaction 1 begins once more to dominate. It’s important for students to recognise that what they see in the classroom is not separate from research. As such, it’s worth pointing out to students that despite the fact that this reaction has been commonly demonstrated since the 1970s, it is still the subject of current research with recent attempts to characterise its mechanism involving at least 30 steps.
Safety
CLEAPSS members should consult CLEAPSS recipe card 63 (oscillating reactions).
Wear eye protection when performing the experiment – the diluted solutions, once prepared for use in the reaction, require no hazard warning.
Disposal
At the end of the demonstration there will be some solid iodine at the base of the vessel that can be reduced with sodium thiosulfate before the solution is poured down the sink with plenty of water.
Tips
Other halide ions can seriously inhibit the reaction – it’s important to use deionised water for making up all solutions. If the starch is not fresh, the results can be disappointing.
If an orange colour fails to appear after one minute, check your hydrogen peroxide – older solutions may not be sufficiently concentrated and new stock may be required.
Stanley Furrow has suggested an alternative recipe using a mixture of IO3– and IO4–, which has a shorter induction period and finishes with iodine in the oxidized form making for an easier clean-up. Although most schools won’t have IO4– salts in stock, these recipes have the additional curiosity benefits of a further transition where the reaction flips one last time up to half an hour after ‘finishing’.
Downloads
Technician notes
Word, Size 53.39 kbTechnician notes
PDF, Size 44.31 kb
References
T S Briggs & W C Rauscher, J. Chem. Edu., 1973, 50, 496 (DOI: 10.1021/ed050p496)
S D Furrow, J. Chem. Edu., 2012, 89, 1421 (DOI: 10.1021/ed200764r)
S D Furrow, R Cervellati & E Greco, React. Kinet., Mech. Catal., 2016, 118, 59 (DOI: 10.1007/s11144-015-0967-4)

























No comments yet