The borax/PVA slime experiment is a lot of fun, but a demonstration of the cross-linking in sodium alginate shows students the creation and destruction of gels through the exchange of ions of different charge. If using food-grade ingredients outside of the lab, the products can be eaten!

Kit
- Sodium alginate (0.5 g)
- Calcium chloride (hydrated) (0.5 g)
- 300 cm3 of deionised water (or low mineral-content drinking water)
- Small sieve or tea strainer
- 1 tsp/5 cm3 measuring spoon
- Dropping pipettes
- Food colouring
- 100 cm3 saturated sodium chloride solution
- Handheld blender to aid dissolution of the alginate
Preparation
Add food colouring to 100 cm3 of deionised water and use this to dissolve the sodium alginate powder. Dissolution is slow, even with the assistance of a magnetic stirrer – using a handheld blender is much quicker. The solution will be full of bubbles so leave it to settle while you dissolve the calcium chloride in a separate beaker containing a further 100 cm3 of deionised water (without colouring).
In front of the class
Adding the coloured alginate solution drop by drop to the calcium bath will instantly create a stream of gel spheres known in molecular gastronomy as ‘caviar’. The caviar can be fished out with a small sieve or a tea strainer and handled by students.
Larger ‘spheres’ can be created by loading a measuring spoon with alginate and gently submerging it over a sieve before tipping out the contents. Five to ten seconds is enough to set the outer surface of the sphere. Lift the sieve and tip the sphere onto a surface or into a bath of deionised water for rinsing.
Lastly, create another large sphere, remove from the water as soon as possible and place into a bath of brine. Over the course of a few minutes, the sphere will rupture and spill its guts into the surrounding fluid leaving only a thin skin behind. This process is much faster if the saturated solution is warm. An hour later, the remaining skin will have almost completely dissolved.
Teaching goal
I normally show this demonstration to students towards the end of a structure and bonding course. The alginate displays properties both of ionic and of covalent materials and students can see how changing the charge on an ion can have an impact on structure.
Sodium alginate is the sodium salt of alginic acid, a polysaccharide used by brown algae to support its cell walls, similar to the way plants use cellulose. It is extracted from seaweed and used widely in food and medicine.
Each repeating sugar monomer in the structure carries a carboxylate anion with a balancing sodium cation.
Once dipped into the calcium chloride solution, calcium ions begin to displace sodium ions from the alginate and exert a greater electrostatic attraction on the carboxylate anions on neighbouring chains. These interactions create cross-links between the chains, forming a gel.
Two types of sugar make up the polysaccharide: (1-4)-linked β-d-mannuronate (M) and α-l-guluronate (G). Repeating G units form a zig-zag structure in which the calcium ions can sit to assist cross-linking.
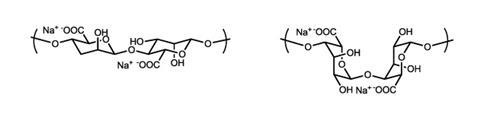
When the gel is placed into brine, sodium ions begin displacing the calcium ions, weakening the cross-links and causing the failure of the sphere’s skin.
The spherification process used above is based on Ferran Adrià’s method. It’s used to deliver intense flavours in cooking. I often perform this experiment outside of the lab with food-grade alginate and calcium chloride (available from online suppliers like Amazon) and allow students to make their own mixtures of caviar to try. Use low mineral-content drinking water for making up the solutions and ensure the spheres are rinsed thoroughly before eating.
Spezi, an odd mixture of cola and orange soda, is sold in Austria and Germany. While many would argue over its culinary merits, it presents students with a caviar challenge: orange soda-flavoured caviar and cola-flavoured caviar can be made and later combined by using the fizzy drink as the base instead of the low mineral-content water. However, one of these will not work without some further thinking (and the addition of some food-grade sodium citrate to increase the pH). Further tips and recipes can be found at www.molecularrecipes.com.
Safety
If students are going to taste the alginate spheres, work outside the lab, use only food-grade or pharmaceutical-grade ingredients, non-laboratory apparatus and low mineral-content drinking water. Students should wash their hands before making and tasting.
Solid calcium chloride is an eye irritant. Bear this in mind using quantities greater than 0.5 g.


















2 readers' comments