Watch this
A demonstration of this experiment for the full explanation. And download the technician’s notes from the Education in Chemistry website: rsc.li/3d2UDw6
I love a demonstration that starts simple but leaves a lot of headroom for adding complexity. In the last Exhibition chemistry we saw ions on the move, but conductivity gives us indirect evidence of the presence of ions in solution, and of their concentrations and even the ways in which water interacts with them. This time we’ll eliminate as many confounding variables as possible to see evidence of the ionic equation of neutralisation, which students may encounter at the 14–16 level. In the next video, we’ll look at the differences in the ways solutions of strong and weak acids behave.
The video contains more detail, explaining why this set-up works. Please do watch it before doing this experiment in front of your class.
Download this
The technician’s notes to help set up and demonstrate this experiment as MS Word or pdf.
A student worksheet to consolidate your students’ understanding of ions and the changes in ionic concentrations observed in an acid-alkali neutralisation, available as MS Word or pdf, and teacher notes with answers, available as MS Word or pdf.
Kit
- 25 cm3 of 0.1 mol dm-3 sulfuric(VI) acid
- 50 cm3 of 0.1 mol dm-3 barium hydroxide (irritating to skin and eyes)
- Approx 225 cm3 of deionised water
- Magnetic stirrer
- Clamp and stand
- Burette and burette clamp
- 500 cm3 beaker
- Conductivity probe (see tips below)
Preparation
Wear eye protection. Load the sulfuric(VI) acid into the beaker and dilute to approx 250 cm3 with deionised water, add a stirrer bead and place on the magnetic stirrer. Clamp a conductivity probe to hold the tip in the middle of the solution and place a burette loaded with the barium hydroxide solution above, ensuring the probe isn’t directly below the jet.
You might be tempted to alter the concentrations or miss out the diluting step. Don’t be.
In front of the class
You may wish to begin by demonstrating the conductivity of deionised water and each of the reacting solutions. Warned about electrocution hazards, students often believe that pure water is a good conductor. However, the LED of CLEAPSS’ conductivity indicator will not light, a conductivity probe should give a value below 10 μS/cm and a multimeter’s resistance reading is likely to give an error because the value is too great. Meanwhile 0.1 M acids and bases will give readings in the tens of thousands of μS/cm. If needed, this step will allow you to choose an appropriate range setting for your probe.
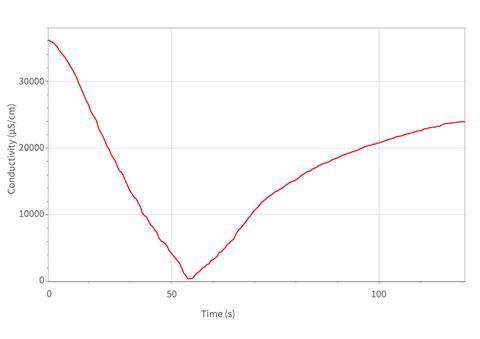
Having introduced the chemistry behind the reaction, simply open the tap and watch for the next two minutes as the burette empties into the solution below. For optimum results with quantitative data, let the tap run half open, as there is a lag of a few seconds between the addition of solution and the completion of precipitation. The conductivity will decrease until half the barium hydroxide has been delivered and where the reading will be almost zero, before it increases until the burette is emptied.
Tips
- Due to the formation of the insoluble carbonate on exposure to air, consider making fresh solutions. CLEAPSS members should consult hazcard 10B for tips on solution preparation and storage. Barium hydroxide octahydrate is often used for demonstrating its endothermic reaction with ammonium thiocyanate and following the pandemic it may not have been recently used – older stock may have substantially reacted with atmospheric carbon dioxide; performed with care, this demonstration will let you know how good your stock is!
- CLEAPSS members should consult guide GL166 for instructions to construct a cheap and effective visual conductivity indicator.
- Multimeters in resistance mode work well too with typical values in the tens of kW range.
- Data collected for this demonstration was from a Vernier conductivity probe.
- Cheap battery-operated digital conductivity probes made for water testing cost less than £10, but the small readout would need a bench cam for groups of students to see.
- Due to ion–ion effects in solution, lower concentrations work better. Probes with ±10 μS/cm resolution are compatible with the concentrations provided above but, if you have at least ±1 μS/cm resolution, consider further diluting acid and base by a factor of ten and benefit from cleaner conductivity curves and still-further reduced hazards (this gives results similar to Figure 2).
Teaching goal
The point of this demonstration is to strengthen students’ mental models of the particles reacting in solution and how this can be represented in chemical equations. A common misconception among students is that ionic substances behave as molecules leading to difficulties understanding dissociation in solution and later, ionic equations.
This demonstration emphasises the ionic and molecular nature of one of the first ionic equations students are likely to meet: that of a neutralisation reaction in which hydroxide ions react with hydrogen ions (equation 1) – or perhaps later, more accurately, hydronium ions (equation 2) – to produce neutral water molecules.
Equation 1: H+(aq) + OH-(aq) → H2O(l)
Equation 2: H3O+(aq) + OH-(aq) → 2H2O(l)
The choice of barium hydroxide and sulfuric(VI) acid allows the change in conductivity of the neutralisation reaction to unfold without confusion from other ions which precipitate out of solution (equation 3).
Equation 3: Ba2+(aq) + SO42-(aq) → BaSO4(s)
Students may be familiar with the insolubility of barium sulfate(VI) as it is the product of the sulfate(VI) anion test with acidified barium chloride.
At its simplest level, a conductivity indicator showing a bulb dimming and brightening may be sufficient, but there is room for exploring the reaction further.
If you simply run 50 cm3 of one 0.1 M solution into 25 cm3 of the other at the same concentration, you will see a curve similar to Figure 1 where the curve dips below a linear trendline before the equivalence point, and then deviates above it afterwards. This is due to dilution factors – the final volume is three times the volume of the initial even if it should have the same number of ions within. These factors are minimised in the demo as described by the initial dilution of the acid.
If you simply run 50 cm3 of one 0.1 M solution into 25 cm3 of the other at the same concentration, you will see a curve dipping below a linear trendline before the equivalence point, and then deviating above it afterwards. This is due to dilution factors – the final volume is three times the volume of the initial even if it should have the same number of ions within. These factors are minimised in the demo as described by the initial dilution of the acid.
The second half of the curve is more stretched as the pressure at the burette jet decreases as the contents above empty out. There is also a lag in the rate of increase of conductivity associated with the delay in precipitation of barium and sulfate(VI) ions out of solution.
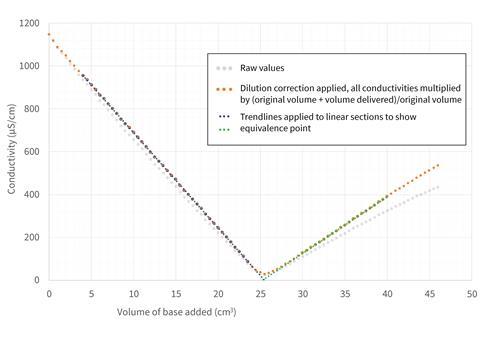
While it would be too time-consuming for a classroom demonstration, keen students could be invited to collect values from a more dilute reaction and plot these against volume rather than time. This gives a fantastic linear relationship (Figure 2), but one question remains: why is the graph still not symmetric? The answer to this will have to wait until the next issue when we introduce some other ions to the mix.
Disposal
Filter the barium sulfate(VI) and dispose of the residue in the normal refuse. You can rinse filtrate down the sink.
Downloads
Conductometric titration: technician notes
Editable handout | Word, Size 0.42 mbConductometric titration: technician notes
Handout | PDF, Size 0.14 mb

























No comments yet