Demonstrations designed to capture the student's imagination
Demonstrations to capture the student's imagination by Adrian Guy of Blundell's School. In this issue: The bizarre oscillating redox reaction between mercury and iron
Redox chemistry, electrode potentials and electrochemical cells are interesting, but can be hard work for sixth form students to understand. This fascinating little demonstration, whilst not the easiest to explain, will always impress your audience.
When an iron nail is placed close the mercury oscillates, 'beats', as if it has a life of its own and you will observe considerable movement or 'beating' if the oscillations are similar to the natural resonant frequency of the droplet. Look more closely and bubbles of gas, relatively rapid oxidation of the nail and fluctuating surface layers of mercury compounds can be seen.

Kit
- mercury
- watch glass or other, flat bottomed, smooth container
- 100 cm3 of 1 M sulfuric acid
- 20 cm3 of 3 per cent (10 vol) hydrogen peroxide solution
- iron nail
- forceps
Safety
1M H2 SO4 is irritating to eyes and skin; eye protection must be worn.
Mercury is toxic by inhalation and there is a danger of cumulative effects. The preparation and use of mercury and mercury compounds must be done in a fume cupboard. A mercury spillage kit should also be available.
You must recycle mercury after use. Mercury should be stored in a plastic bottle and transferred to and from the beaker over a wooden or plastic tray that could contain any spills. Rings should be removed or protective gloves worn as mercury amalgamates, eg with gold, turning rings 'silvery'.
Procedure
Working in a fume cupboard, add enough mercury to your watch glass to form a drop approximately 15 mm in diameter. Pour sulfuric acid over the mercury until it is completely immersed in acid and add the hydrogen peroxide solution.
Place an iron nail into the mixture and use the tweezers to arrange the tip of the nail so it is almost touching the mercury. Be patient and adjust the distance between the tip of the nail and the drop of mercury until the oscillations begin.
Special tips
- If the oscillations are too fast, move the nail a fraction away from the mercury.
- Tapping the container to knock the mercury into the nail can start the oscillations.
- Be patient, it can take a few seconds for the reaction to start.
- Don't always place the nail in the middle of the mercury drop: placing the tip of the nail near one end can produce some more impressive oscillations and resulting shapes.
- A few sodium chromate(VI) crystals could be used instead of the hydrogen peroxide, however they should be avoided if possible as they are very toxic.
Theory
The theory of this reaction is not well understood, but a sensible mechanism involves hydrogen peroxide oxidising the mercury to mercury(I). The oxidised mercury combines with the sulfate ions from the acid on the metal surface forming a layer of solid mercury(I) sulfate. This lowers the surface tension and allows the drop to flatten.
On approach or contact with the iron nail an electrochemical redox reaction takes place oxidising the iron to iron(II) and reducing the mercury(I) back to metallic mercury. This restores the surface tension and shape of the drop. The net result is that the nail is oxidised by the oxidising agent.
Regardless of the exact mechanism, conditions and oxidation states, it is sufficient to use the standard electrode potentials for mercury and iron to explain the redox reaction between the nail and mercury sulfate and in doing so make an educationally valid conclusion. Assuming standard conditions
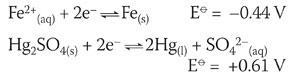
The cell diagram can be written as:
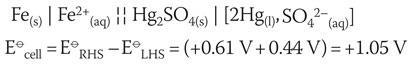
The calculated E⦵cell indicates a spontaneous reaction. The anticlockwise rule can also be applied where Fe(s), being the most negative, moves left forcing the Hg2 SO4 (s) equilibrium to the right.
A similar cell can be formulated for the oxidation of the mercury by hydrogen peroxide:
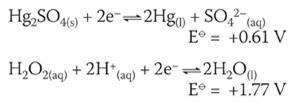
E⦵cell for the reaction between Hg(l) and H2 O2 (aq) is +1.17 V.
Safety
1M H2 SO4 is irritating to eyes and skin; eye protection must be worn.
Mercury is toxic by inhalation and there is a danger of cumulative effects. The preparation and use of mercury and mercury compounds must be done in a fume cupboard. A mercury spillage kit should also be available.
You must recycle mercury after use. Mercury should be stored in a plastic bottle and transferred to and from the beaker over a wooden or plastic tray that could contain any spills. Rings should be removed or protective gloves worn as mercury amalgamates, eg with gold, turning rings 'silvery'.









No comments yet