Ida Emilie Steinmark finds out how new research may boost catalytic performance
We know very little about Elizabeth Fulhame. We know she was married to a physician. We know she was interested in staining cloth. And we know this interest led her – against her husband’s advice – to experiment and ultimately publish her discovery of catalysis in 1794. This discovery has revolutionised society in a way no other chemical concept has. Her brilliance, and sex, astonished the scientific community of the day. More than anything she showed that the best scientific viewpoint can sometimes come from the most unexpected angle.

In your class
Download the text of this article (MS Word or pdf), and all the teaching ideas as a single file (MS Word or pdf).
Download the text of this article, and all the related teaching resources, worksheets and experiments from the Education in Chemistry website: rsc.li/EiC617uc
Catalysis is a core topic in secondary school chemistry. Many of the catalysts pupils study will appear to belong to old-fashioned chemistry (eg the Haber and Contact processes) and students may consider enzymes as firmly within a box marked ‘biology’. This article provides a useful introduction to the most up to date research in catalysis that shows catalytic processes are constantly improved and evolved. There is a bewildering array of different demonstrations and whole class investigations for this topic. So, in the attached teaching ideas we have signposted some favourites, including tried and tested ones from Learn Chemistry.

Specification links
Download a list of specification points this article supports from the Education in Chemistrywebsite: rsc.li/EiC617uc
Fast forward more than 200 years, and unexpected angles still drive the field of catalysis onwards. Some of the best progress takes inspiration from other fields, trying their methodologies and borrowing their perspectives. In fact, many of the techniques steering catalysis research today are not from the classical chemistry toolbox. Rather, the new toolbox is filled to the brim with biophysics, computer simulations and molecular biology.
Like a living object
‘First of all, you see right away that cell dimensions are on the same scale as some catalyst particle dimensions – from a few micrometres to tens of micrometres,’ Bert Weckhuysen, professor of inorganic chemistry and catalysis at Utrecht University, points out. He thinks a catalyst particle can be studied almost as though it’s a living object. It’s an analogy he believes goes far; even the heterogeneity inside a catalyst can correspond to the different organelles inside the cell. ‘What is inside the catalyst is heterogeneous, there are multiple components in it,’ he says. ‘This you can also compare to a cell’s organelles – you have the mitochondria and the nucleus. So the objects which are in a cell and the objects you find in a catalyst particle go from atoms to molecules to nanometre structures up to micrometre structures.’

Busting gender myths
Draw a scientist homework, primary and ages 11–14
The first paragraph of this article is powerful for busting gender myths in STEM. Elizabeth Fulhame’s work was almost forgotten until recently. A great first homework for ages 11–14, or even primary pupils, is to get them to draw a scientist. When collected in, discuss what stereotypes have arisen. Despite the progress made in the visibility of women in STEM in recent years it is still likely that pictures will be caricatures of white, old men with crazy hair and white coats.
Follow up with some display work showcasing the diversity of science and scientists beyond the famous men represented in textbooks. Students can turn scientist profiles into LEGO® minifigures.
This year scientists took to Twitter to show the range of their work. The hashtag #reallivingscientist when combined with #dresslikeawoman brings up a wide range of female scientists. In addition, ‘Not all chemists wear white coats’, and ‘Faces of Chemistry’ within Learn Chemistry are useful research resources.
Download a LEGO® minifigure handout for students to design their scientist profiles (MS Word or pdf).
This year scientists took to Twitter (bit.ly/2fCHQD2) to show the range of their work. The hashtag #reallivingscientist when combined with #dresslikeawoman brings up a wide range of female scientists. In addition, ‘Not all chemists wear white coats’, and ‘Faces of Chemistry’ within Learn Chemistry are useful research resources: rsc.li/whitecoats; rsc.li/faceschemistry.
Download a LEGO® minifigure handout for students to design their scientist profiles from the Education in Chemistrywebsite: rsc.li/EiC617uc
But it is not only the connection in size and structure that conceptually links catalyst particles and living cells. ‘Catalyst particles are also dynamic,’ says Bert. ‘They also change. They also function. Of course a catalyst is not alive in terms of reproducing itself, but it has functionalities that remind us of what happens in life.’ All these features mean many tools used by biophysicists and biologists are also useful in catalysis science – especially tools that bring a hefty zoom function and the ability to follow a sample over time. For these reasons, microscopy and spectroscopy have started to creep into the catalysis chemist’s repertoire.
If you can get close enough to the catalyst to see what’s going on, both in this moment and over the course of a reaction, you can gain valuable information about the catalytic system. If, for example, the catalyst stops working, the equivalent of a massive pair of binoculars directed straight at the crime scene is a big help. ‘In presentations, I often mention Hercule Poirot, the detective,’ says Bert. ‘It’s a whodunnit. Who killed my active site? You have to answer the questions: Where did it happen? What was the weapon? Who was it?’
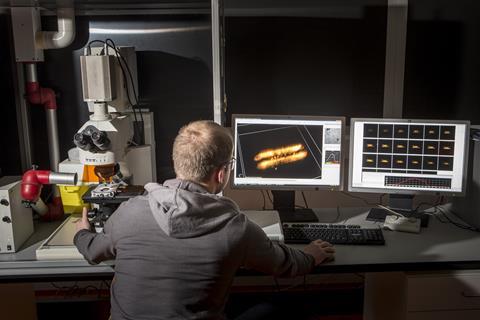
The use of large microscopes is a neat way to solve mysteries like this. Fluorescence microscopy, which works by collecting the light coming off small glowing molecules, is already widely used in biology and biophysics. This technique has helped visualise how catalytic reactivity can vary between different parts of the catalyst and how activity can appear in zones. With electron microscopes, which provide even better resolution, researchers can even study tiny structural changes on the surface of catalyst nanoparticles. ‘It shows visually what people have already been thinking of as possibilities,’ says Bert. ‘But now you see it! The nice thing is that we can show an image and say “this is the case”.’
One conclusion from spying on them at work is that catalyst materials are more heterogeneous than previously assumed. Everything from structure to reactivity can vary throughout the catalyst material, and this can change markedly with time. ‘People think their catalyst is homogeneous: uniform in space,’ says Bert. ‘But every time I have a new tool in my toolbox that gives more powerful resolution, I see a new heterogeneity popping up. Some of this gives leads for better design.’
Model-made progress
New and improved design is the reason industry in particular is so interested in new catalysis research. A better catalyst means a more efficient reaction, often with less waste and under less extreme conditions too. Another helpful approach to reaching this goal is modelling. ‘Almost all chemical companies have modelling teams,’ says Richard Catlow, professor of catalytic and computational chemistry at Cardiff University, ‘because they know modelling produces information that is valuable in the development of their catalysts.’ This is no surprise, as a new, superior catalyst could make its developers some serious money.

Catalysis in exams
Exam style practice questions, ages 14–16
Catalysis is a commonly assessed concept in GCSE and National 5 examinations. This resource includes a number of different question types for the topic and is suitable for a classwork or revision exercise. Also included is a ‘level of response’ question, similar to those included in the new GCSE assessments.
Download the worksheet of six exam style questions for students (MS Word or pdf) and the answers sheet (MS Word or pdf)
Download the worksheet of six exam style questions for students and the answers sheet from the Education in Chemistrywebsite: rsc.li/EiC617uc
While biophysical methods are helping chemists light up and see properly their own catalysts, computer-savvy modellers are giving catalysts their own treatment. Mirroring a field-wide trend of increased use of simulation, computational chemists are finding themselves in great demand within all catalysis research. ‘Almost all major groups in catalysis will have someone, or maybe even a team, doing modelling work,’ says Richard, highlighting how extensively modelling is finding use within a previously very experimental field. ‘It’s simply one of the oldest scientific activities – model building – using contemporary technology. And of course, it goes hand in hand with experiment.’
Although it is an old discipline, today’s models have a lot to bring to the table. ‘Computational methods now are extremely powerful,’ says Richard. ‘What you’re doing is building up an understanding of catalysts on the molecular level, and you really need to know [this] if you’re going to improve them and optimise their performance.’ Crucially, insight gained from modelling can be coupled with experiments to improve upon existing catalysts. ‘You can understand, for instance, the relationship between the active site of the catalyst and the mechanism,’ he explains. ‘Once you [do that], you can perhaps try and modify the active site, try and control it, try and control the distribution of active sites to improve the efficiency of the catalyst.’
To make a good catalyst, chemists also have to consider not just how well it works when it works, but how and why a catalyst starts to switch off and become unusable. In other words, as catalysts have a certain lifetime, how could that be stretched further? According to Richard, this part of catalysis isn’t given as much attention as it deserves. ‘Deactivation is a key process,’ he says. ‘With modelling and experiment, we can now try and understand the atomic mechanism that underlies deactivation, and then try and prevent it or make it happen more slowly.’
Modelling isn’t just used to optimise existing systems, but also to find new and better ones – and not just better for the reaction but also for the environment. One key industrial process is the production of polyvinyl chloride (PVC), a type of plastic used for an enormous range of products, from bottles to pipes. Making PVC involves converting acetylene into vinyl chloride. Most of the industrial plants carrying out this process have used a catalyst based on mercury, which is toxic and polluting. Richard’s colleague, Graham Hutchings, developed an alternative method that instead uses a more environmentally benign gold catalyst. ‘[We] used both computer modelling and synchrotron radiation to how this actually worked,’ Richard explains. ‘That technology is new chemistry, and is now being implemented in plants in China.’
A bio future
Efforts to replace catalytic processes with more sustainable ones have been particularly successful in the pharmaceuticals industry. And increasingly, many of their new catalysts are biological catalysts, or biocatalysts. Better known as enzymes, these are borrowed from molecular biology rather than from the classic chemistry toolbox.

Decomposition of hydrogen peroxide demonstration
Comparing catalysts with elephant’s toothpaste, ages 11–14 and 14–16
The decomposition of hydrogen peroxide is a classic demonstration which can be used to compare catalyst effectiveness when time and logistics mean a whole class investigation is impractical. The reaction forms the basis of the demonstration known as ‘elephant’s toothpaste’. Mix hydrogen peroxide in a measuring cylinder with washing up liquid and add a catalyst. The hydrogen peroxide decomposes to oxygen and water, producing bubbles which make the mixture shoot up the measuring cylinder.
Using the above article, ‘Catalysts get helping hands’ as a stimulus, compare traditional chemical catalysts (like potassium iodide, manganese dioxide and iron(III)oxide) and enzyme based catalysts found in yeast, lettuce, potato, liver, and blood.
Emphasise to pupils that the hydrogen peroxide is decomposing without any catalyst, but very slowly. The catalysts added just speed it up. A common misconception is that added catalysts make the reaction happen, rather than making an existing reaction happen quicker (in this case, much quicker). This demonstration also provides a useful opportunity to revisit the test for oxygen: a glowing splint placed near the top of the foam should relight.
This demonstration is simple and effective, but it is worth practising beforehand as hydrogen peroxide can be temperamental. Quantities found in guidance documents may need adjusting depending on the age of your hydrogen peroxide and where it has been stored.
Suggested lesson order
- Demonstrate the reaction and prompt pupils to suggest ways the best catalyst could be judged.
- Carry out the series of reactions with pupils recording their observations on the pupil sheet provided as you go along.
- Give pupils copies of the article ‘Catalysts get helping hands’ to help them complete the follow up questions.
Further guidance
The Decomposing hydrogen peroxide article and Learn Chemistry give further guidance for this demonstration. Additionally, Learn Chemistry has a class practical resource for detecting the presence of enzymes in liver, potato and celery.
Download the teacher notes (MS Word or pdf) and student handouts (MS Word or pdf)
The Decomposing hydrogen peroxide article (rsc.li/hydrogenperoxidedemo) and Learn Chemistry (rsc.li/H2O2decompose) give further guidance for this demonstration. Additionally, Learn Chemistry has a class practical resource for detecting the presence of enzymes in liver, potato and celery: rsc.li/detecting-enzymes-practical.
Download the student handouts from the Education in Chemistrywebsite: rsc.li/EiC617uc
‘In pharmaceuticals, one of the most important things is enantioselectivity,’ explains Roger Sheldon, professor emeritus of biocatalysis and organic chemistry at Delft University in the Netherlands, and known as a founding father of green chemistry. Enantioselectivity is the ability to preferentially produce a specific chiral product, which is usually very hard to do. But since biology is overwhelmingly chiral, enzymes do this easily.
‘A lot of pharmaceutical companies used to use things like rhodium-catalysed asymmetric hydrogenation,’ says Roger, ‘but now they’re switching to enzyme-catalysed reactions because they’re just better.’ He’s right: biocatalysts are more cost-effective and more environmentally friendly. In fact, they’re inherently sustainable as they’re derived from renewable resources and are both biodegradable and non-toxic.
Catalysts for asymmetric processes have bulky groups in just the right places so the substrate can only access the active site in one specific way. This ensures the selectivity. Unless an asymmetric catalyst is discovered by chance, it must be designed to be the correct shape. ‘The big difference with enzymes,’ says Roger, ‘is the enzyme will have evolved to create that bulkiness where it is necessary. The enzyme itself will work it out.’
But it’s not always that easy. Many reactions are unlikely to switch to enzymes any time soon, because they need to be run at high temperatures or in harsh solvents that would render the enzyme immediately defunct. Even though some reactions are still off limits, it’s becoming easier to make a biocatalyst work under specific industry conditions by modifying the enzyme, at least if you have a helpful molecular biologist on your team.
Magnificent mutants
One modification approach is called directed evolution. ‘Of course, the [original] enzyme was evolved in nature,’ Roger explains. ‘It took millions of years for it to get where it is today. With the help of directed evolution, you can do this in weeks or maybe even less. The big point is that you can write down your wish list and say “this is what I want the enzyme to do”, and then you can evolve it to do that – within reason!’ During directed evolution, a gene is copied and made to mutate many times through a process called random mutagenesis. Then the new mutants are sliced up and put back together in different ways, to make even more new, random genes. The result is a vast library that can be screened for the required functionality.
Scientists are aware they have only discovered less than 1% of all existing enzymes
Today, even more sophisticated methods have been developed that combine directed evolution with the computational design approaches in ordinary catalysis. By using computer simulations, scientists can make more focused libraries of mutants, rather than random mutants, so they don’t need to screen enormous numbers of enzymes. ‘That has been a big breakthrough,’ Roger says. ‘The combination of computer-aided design and random mutagenesis has changed biocatalysis.’
The arsenal of useful enzymes could be broadened further by yet undiscovered natural enzymes. According to Roger, scientists are aware they have only discovered less than 1% of all existing enzymes. As such, scientists suggest simply looking through the 30,000 sequenced and publicly available microbial genomes to find new enzymes for the next new dream reactions.
Dreaming is indeed what scientists do best, whether it’s about a ground-breaking, sustainable reaction, the first atomic-level movie of a catalyst surface or a new way to stain cloth. ‘I’m very fortunate that I’m in a field where everything has direct applications for our society,’ says Bert. ‘But we should also teach students to enjoy the science. Having tremendous impact in sustainability is a very nice outcome of this science, but it remains beautiful.’ Like Elizabeth Fulhame, today’s catalyst chemists can’t help themselves – they just have to give it a go.
Downloads available for this article
- Full article, without resources or notes (MS Word or pdf)
- All teaching ideas in a single file (MS Word or pdf)
- Specifications links (MS Word or pdf)
- Minifigure student handout (MS Word or pdf)
- Exam style 14–16 questions (MS Word or pdf)
- Exam style 14–16 questions answers (MS Word or pdf)
- Decomposition of hydrogen peroxide teacher notes (MS Word or pdf)
- Decomposition of hydrogen peroxide student handout (MS Word or pdf)
Teaching resources by Kristy Turner, a school teacher fellow at University of Manchester and Bolton School, UK
Downloads
Full article
Word, Size 58.61 kbFull article
PDF, Size 61.3 kbAll teaching ideas
Word, Size 61.5 kbAll teaching ideas
PDF, Size 91.04 kbSpecifications links
Word, Size 55.14 kbSpecifications links
PDF, Size 49.77 kbMinifigure student handout
Word, Size 0.15 mbMinifigure student handout
PDF, Size 94.3 kbExam style 14–16 questions
Word, Size 55.61 kbExam style 14–16 questions
PDF, Size 52.81 kbExam style 14–16 question: answers
Word, Size 0.28 mbExam style 14–16 question: answers
PDF, Size 0.12 mbDecomposition hydrogen peroxide: teacher notes
Word, Size 54.47 kbDecomposition hydrogen peroxide: teacher notes
PDF, Size 0.11 mbDecomposition hydrogen peroxide: student handout
Word, Size 54.46 kbDecomposition hydrogen peroxide: student handout
PDF, Size 46.73 kb









No comments yet