Certain brightly coloured frogs of South America, Australia and Madagascar hold a sting in their skin - they exude deadly alkaloids when put under stress or attack. The natives of these lands called them 'poison dart frogs,' and used them for hunting and defence.
-
American chemist John W. Daly spent a lifetime finding out which alkaloids were responsible for the venom of poison dart frogs
- Daly discovered a host of alkaloids, some of which went on to be synthesised by other chemists and used as pharmaceuticals

Sixteenth century European travellers to South America returned with many stories, one of which was that the native South Americans were using arrows tipped with poisons for hunting and defence. Known locally as 'ourari', 'urari' and 'urali', these poisons, which we now know as curare,were a dried extract from the plant Chondodendrum tomentosum found in these regions.
By the mid-1800s, scientists, notably French physiologist Claude Bernard, had confirmed by experiment that curare worked by blocking the transmission of nerve impulses to the muscles.1 Injection of the arrow poison into the bloodstream caused death by respiratory failure because the chest and abdominal muscles became paralysed. Indeed, these molecules are only effective by injection into the bloodstream and cannot be absorbed from the digestive system, a point which was important to the native Indians who used the poisons for hunting for food.
We now know that curare acts as an antagonist to the chemical signaling molecule, or neurotransmitter, acetylcholine. Normally, when acetylcholine is released from a neuron it binds to a specific receptor (the nicotinic receptor) on another cell in muscle fibres. A transmembrane channel opens and sodium ions enter the fibre of the muscle, and this triggers muscular contraction. Curare stops the channel opening by binding to the acetylcholine receptor and now the muscle cannot contract, resulting in paralysis.
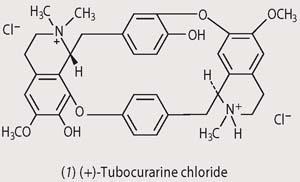
Although samples of the poisonous material were brought back to Europe in the 18th century, it was not until 1935 that Harold King at the National Institute for Medical Research in London, isolated the most potent extract, (+)-tubocurarine chloride (1), from a dried museum sample and established its structure.1 By the 1940s tubocurarine chloride was being used in abdominal surgery as a muscle relaxant to reduce the amount of anaesthetic required. (Twentieth century chemists soon realised that the presence of a quaternary nitrogen atom was essential for a species to have muscle relaxant properties, and went on to identify other compounds with curare-like effects, notably succinyl chloride.) 2
Poison arrow frogs

While curare is the most well-known 'arrow poison', there were others, even more toxic than curare, that were used by the South Americans to coat their arrows. In his 1825 book of reminiscences of his travels in Colombia, Captain Charles Stuart Cochrane recorded the Chocó Indians' use of venom obtained from small yellow frogs to provide a highly toxic tip to their arrows:
A tiger when hit, runs ten or a dozen yards, staggers, becomes sick, and dies in four or five minutes. A bird is killed as with a bullet.
Brightly coloured Columbian frogs, of the genera Phyllobates, Dendrobates, Epipedobates and Minyobates, and Australian frogs of the genus Pseudophryne, as well as mantellid frogs of Madagascar, secrete toxins from their skins when stressed or under attack. They are known collectively as 'poison dart frogs'. Natives force the reptiles to release their poisons by passing an arrow down their throats and out of one leg. The toxins have noxious effects on buccal (mouth) tissue - predators have been seen to pick up these frogs in their mouths, and rapidly drop them.
Over the past two centuries, scientists have puzzled over how these frogs produce such venomous molecules, whether all the toxic substances are the same, and just how toxic they are. In the past 40 years they have begun to determine the structures of these molecules and shed some light on how they work. Much of this work was done by John W. Daly of the National Institutes of Health in the US.
Batrachotoxin
By far the most poisonous of these molecules are the steroid alkaloid batrachotoxin (2) and the related homobatrachotoxin (3). Around 10 times more toxic than curare, they are found in Phyllobates terribilis, and to a lesser extent in P. bicolour and P. aurotaenia - the yellow frogs used by the Chocó Indians. When these frogs are stressed (in pain or threatened) they exude these toxins from glands in their back and from behind their ears. One frog contains, on average, 1100μg of batrachotoxin and the the lethal dose for humans is 1-2 gμkg-1, ca 100 micrograms in an 11 stone person (less than a hundredth the size of a grain of rice).
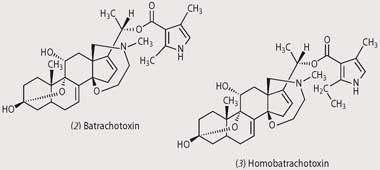

Batrachotoxin exerts its toxic effect by increasing the permeability of the outer membrane of nerve and muscle cells to sodium ions. An influx of sodium ions into the cell causes an irreversible electrical depolarisation, which blocks nerve signals that would normally cause the muscle to relax. Certain cells within the heart are very sensitive to this increase in Na+, resulting in heart arrhythmias and ultimately cardiac failure. The frogs are not affected because they have a modified sodium channel protein in their nerves and muscles, to which batrachotoxin cannot bind.
Poison dart frogs in general do not synthesise toxic molecules such as batrachotoxin, but obtain them from their diet. Evidence for this comes from the fact that Phyllobates terribilis born in captivity don't have batrachotoxins in their skins; and of those poison frogs caught in the wild, the amount of toxin in their skins often diminishes with keeping. Moreover, if batrachotoxin-free offspring are fed batrachotoxin in their diet, they accumulate it in their skins. The alkaloid is believed to be in certain beetles.
Epibatidine
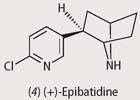
In 1974 Daly isolated the alkaloid epibatidine (4) from the venom of Epipedobates tricolor frogs of Ecuador, but it was nearly 20 years before he was able to work out its structure. Deforestation led to the frog being an endangered species, and frogs bred in captivity did not contain epibatidine. With just a 1 mg sample available, Daly had to wait until more sophisticated spectroscopic methods allowed him to deduce the structure. Once the structure was known, organic chemists soon rose to the challenge of its synthesis.
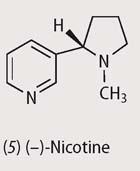
Epibatidine, which exists in Nature as the (+)-isomer, is a strong nicotine agonist - ie a molecule which, like nicotine (5), binds to nicotinic receptors, and blocks pain; this alkaloid is some 200 times more potent than morphine. 3 Since it does not act through the opioid receptor, chemists reasoned that it might be useful as a non-addictive painkiller.
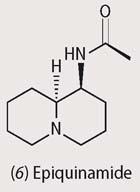
However, epibatidine was too toxic to be used (the lethal dose is ca 2 mg), but it became the lead compound in the synthesis of hundreds of molecules with related structures that were tested for drug activity. One of these, ABT-594 showed great promise as a non-addictive painkiller, but it had side effects that prevented its medical use. Epiquinamide (6), a quinolizidine alkaloid more recently obtained from skin extracts of Epipedobates tricolor, is also a nicotinic agonist and may hold more promise; its backbone resembles acetylcholine, the natural ligand for the nicotine receptor.4
We still don't know how the frogs obtain the epibatidine, since they do not make it when removed from the forest. Their diet, again, could play a role - arthropods among leaf litter on the forest floor are a likely possibility, as with other frogs.
Other alkaloids
Found quite widely in 'poison dart frogs', pumiliotoxins (7) are a family of bicyclic alkaloid molecules some 100 to a 1000 times less toxic than batrachotoxin. Pumiliotoxin A is the most poisonous of these compounds, and like batrachotoxin, opens up transmembrane sodium channels, which leads to paralysis.
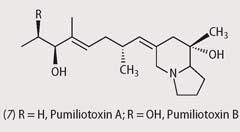
These compounds were first identified in the strawberry poison frog, Dendrobates pumilio (recently reclassified as Oophaga pumilio), common to Costa Rica, and more recently in Madagascar frogs of the genus Mantera and in Australian Pseudophryne frogs.
The widespread distribution of these alkaloids suggests that the frogs' food source must itself be widespread. Indeeed, pumiliotoxins are found in formicine ants,5 specifically B. longicornis and P. steinheili which have also been found in the stomach contents of the dendrobatid frog, Oophaga pumilio.
More recently, examination of a wide range of Oribatid mites collected throughout Costa Rica and Panama has identified around 80 different alkaloids, including pumiliotoxins, which are also found in poison frogs, plus around 40 other alkaloids, of as yet unknown structure that have not yet been identified in frogs. Scheloribatid mites are also a source of pumiliotoxins and other alkaloids.6
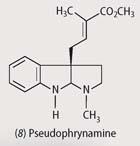
Australian mycobatrachid frogs of the genus Pseudophryne contain two kinds of alkaloids in their skin: pumiliotoxins, and indolic alkaloids, eg pseudophrynamines (8). Unlike the pumiliotoxins, which the frogs acquire in their diet, they can synthesise pseudophrynamines.7
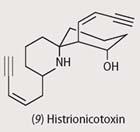
The major alkaloid found in the Columbian poison frog, Dendrobates histrionicus, is histrionicotoxin (9). Histrionicotoxins are potent nicotinic non-competitive antagonists, blocking the acetylcholine binding site.
The distance between the NH and OH groups is similar to that in acetylcholine, and this has been linked with histrionicotoxin's activity.
And finally
The research used to characterise these substances, often on an incredibly small scale, has been persistent and painstaking (see Box). The molecules used by these frogs in their defence vary considerably in both their means of action and structures, many of the latter being quite novel.
In several cases, the ultimate source of the toxic molecules still remains to be discovered. Nature is, as usual, the best and most inventive organic chemist around, and may yet have given us the lead in finding new and useful pharmaceuticals.
Dr Simon Cotton is a chemistry teacher at Uppingham School, Uppingham, Rutland LE15 9QE.
Acknowledgements
I thank the referees, and Dr John Emsley and Professor James Anderson, for helpful comments. John W. Daly died on 5 March 2008; this article is offered as a tribute to a great chemist.
Further Reading
- J. W. Daly, J. Nat. Prod., 1998, 61, 162.
- J. W. Daly et al, Nat. Prod. Rep., 2000, 17, 131.
- S. Feldman, Poison arrows. London: Metro Books, 2005.
- M. Plotkin, Medicine quest: in search of Nature's healing secret s. New York: Viking, 2000.
- J. G. Walls, Jewels of the rainforest: poison frogs of the family Dendrobatidae. New Jersey: T. F. H. Publications, 1994.
References
- J. Mann, Chem. Br., 1998, 25 (5), 478.
- A. T. Dronsfield, M. Hill and J. Pring, Educ. Chem., 2003, 40 (3), 74.
- J. B. Traynor, Br. J. Anaesthesia, 1998, 81, 69.
- R. W. Fitch et al, J. Nat. Prod., 2003, 66, 1345.
- R. A. Saporito et al, Proc. Natl. Acad. Sci. USA, 2007, 104, 8885.
- W. Takada et al, J. Chem. Ecol., 2005, 31, 2403.
- B. P. Smith et al, J. Nat. Prod., 2002, 65, 439.






No comments yet