From a chance discovery by a French saltpetre manufacturer, iodine celebrates 200 years of use in industry and medical science in 2011
-
200 years ago there was a race to confirm the disovery of iodine
-
Today it can help to protect our bodies against radiation damage
This year is the bicentenary of the discovery of iodine by Paris saltpetre manufacturer Bernard Courtois, although it remained officially unrecorded for nearly two years. Fortunately, when details began to emerge late in 1813 the famous chemist Sir Humphry Davy and his assistant the young Michael Faraday were in Paris on a continental tour. Despite the Napoleonic wars (1796-1815) between Britain and France, they had been granted passports by the French government in recognition of Davy's fame. Faraday's journal for 1 December reads:
On this and the preceding day Sir H. Davy made many new experiments on the substance discovered by
M. Courtois ... M. Clément has lately read a paper on it at the Institute, in which he says it is procured from the ashes of seaweeds by lixiviation and treatment with sulphuric acid: he conceives it to be a new supporter of combustion.
The discovery of this substance, in matters so common and supposed so well known, must be a stimulus of no small force to the inquiring mind of modern chemists.1

Discovery confirmed

Faraday describes Nicolas Clément's announcement to the French Institute on 29 November of the discovery in kelp of a 'curious substance with a metallic appearance giving a superb violet vapour when gently heated.' Humphry Davy had received a specimen of this on 23 November when the physicist Ampère visited him with the chemists Charles Désormes and his son-in-law Clément. The day after Faraday's journal entry, the official newspaper Le Moniteur reported on Clément's paper. The news was out at last.
At Clément's request, the French chemist Joseph Louis Gay-Lussac had also studied the substance. He addressed the Institute on 6 December, calling it iode (from the Greek iodes, meaning violet-colour) and concluded it was an element analogous to chlorine.2 Humphry Davy had reached the same conclusion but his letter, dated 11 December, was read at the Institute a week after Gay-Lussac's paper.3 Davy claimed precedence in this discovery, stating in his letter that he had informed the Institute's secretary M.Cuvier 'eight days ago' of his conclusions. Although Davy and the French chemists disputed who had first identified iodine as an element, all concurred that its discoverer was Bernard Courtois in 1811.
Saltpetre production
During Bernard Courtois' youth in Dijon, the saltpetre industry in France underwent many changes. Gunpowder, urgently required for use in cannons and muskets, consists of 75% saltpetre (nitre, KNO3), which, to be effective, had to be uncontaminated by deliquescent sodium or calcium nitrate. Until the loss of French India, natural saltpetre had been imported from the subcontinent or collected by itinerant saltpetre officials from the KNO3 -rich efflorescence that formed on cellar and stable walls. The industry was nationalised in 1775 and saltpetre began to be produced in locally-organised nitriaries or nitre beds. These were heaps of decomposing nitrogenous organic matter (animal and vegetable material, soil, manure, straw, and wood ashes containing potash (K2 CO3)). The nitriaries were kept under cover, moistened with urine and occasionally agitated with twigs, so air could penetrate. After about a year the beds were watered. The run-off contained sparingly-soluble potassium nitrate, which would crystallise out. This was repeated yearly until the beds were exhausted. Further saltpetre was produced from sodium nitrate and other nitrates in the mother-liquors, such as calcium and magnesium, by double decomposition using potassium salts from wood ashes:
NaNO3 + KCl → KNO3 + NaCl
Ca(NO3)2 + K2CO3 → CaCO3 + 2KNO3
Using kelp
A shortage of suitable wood ashes for saltpetre production at the time of Courtois' discovery meant that ashes of brown seaweed (kelp) were being used instead. The kelp industry began in France in the early 18th century and spread rapidly to places along the Western coasts of Europe, particularly in Scotland, Norway and Ireland. Kelp was a main source of soda (Na2 CO3) for the growing glass, soap, pottery and textile industries before the Leblanc process provided cheap soda. It was cut and dried on the shore then arranged in hollowed-out pits carpeted with stones. Fires of dry gorse lit over the seaweed slowly fused the masses of kelp, which were then cut into blocks. The kelp contained KCl, K2 SO4 and soda (Na2 CO3), which Courtois extracted at his factory to manufacture saltpetre. After lixiviation (aqueous extraction) he evaporated the solutions, to yield first a deposit of sodium chloride, then the potassium salts, and finally, crystalline soda.
Properties of iodine determined by Bernard Courtois5
- at ordinary temperatures the substance is a black metallic-like solid
- it sublimes giving a violet vapour on gentle heating, to 70°C
- at red heat it does not react with carbon or with oxygen
- it is changed on reaction with hydrogen and an acid is formed - thought to be muriatic acid (HCl). Similarly with phosphorus
- it attacks metals directly and combines without effervescence
- it reacts with the metal oxides forming compounds soluble in water
- with ammonia a precipitate is formed which explodes with a bang when dried
The fortuitous discovery of iodine
A letter Humphry Davy wrote to the Royal Society on 10 December 1813 from Paris provides some details of how Courtois made his discovery:
This substance was accidentally discovered about two years ago by
M. Courtois. In his process for procuring soda from ashes of sea weeds he found the metallic vessels much corroded; and in searching for the cause of this effect, he made the discovery. The substance is procured from the ashes, after the extraction of carbonate of soda, with great facility, and merely by the action of sulphuric acid: - when the acid is concentrated, so as to produce much heat, the substance appears as a vapour of a beautiful violet colour, which condenses in crystals having the colour and lustre of plumbago. 4
The corrosion in the pots was due to sulfurous and other salts remaining in the mother-liquors. When the observant Courtois investigated this he used sulfuric acid and noticed the violet vapour of iodine rising from iodides of sodium and potassium in the residue:
2NaI + 2H2SO4 → I2 + Na2SO4 + SO2 +2H2O
He studied this very interesting substance himself before asking the chemists Clément and Désormes to continue the work due to the demands of his saltpetre business. Other scientists soon followed Gay-Lussac and Davy's brilliant studies on the new element. Before long the general chemistry of iodine and its compounds was well known. Following research in the 1820s, iodine became valued for its medicinal properties and this led to the French Institute honouring its discoverer, Bernard Courtois, with a prize in 1831.
Medical applications
There were no immediately obvious uses for this element. Its applications in photography and dyestuffs date from the later years of the 19th century. However it was soon adopted by some physicians, and in 1835 it was being prescribed, hopefully rather than scientifically, for
-
various forms of scrofula (a skin disease, often caused by tuberculosis)
-
ovarian dropsy (swelling of the abdomen related to advanced ovarian cancer)
-
strictures of the urethra
-
hypertrophy of the heart and mammae (ie breasts)
-
obstruction of the Eustachian tubes
and indeed many more conditions. None of these stood the test of time.
There were three indications where it was effective, leading to its widespread use in the later 19th century: in killing germs; in treating goitre; and in relieving some symptoms of syphilis.
As a disinfectant
'Germs' became prominent in the early 1860s following the work of Louis Pasteur and later, Joseph Lister. The latter surgeon used phenol solution to sterilize skin, instruments and dressings, significantly reducing post-operative morbidity and mortality caused by infection.6 This success prompted a search for other antiseptics.
Tincture of iodine (a 2-3% solution of the element in alcohol) had been used before Lister's discovery to slow the putrefaction of meat, but it was expensive compared to phenol. Nevertheless it was used to sterilize surgical sutures (silk threads used for stitching wounds) from about 1870. Its relative expense did not prevent it becoming popular in home medicine chests, where it was poured onto wounds in the hope of preventing infection. The intense stinging pain it caused probably convinced the unhappy patient that good was being done. In truth, cleaning the tissues with soap and water would have served equally well. Iodine certainly kills bacteria in test tubes. On open wounds it destroys healthy tissues too and probably delays healing overall (and increases scar tissue formation). Today the tincture is most frequently used for 'emergency' sterilization of drinking water.
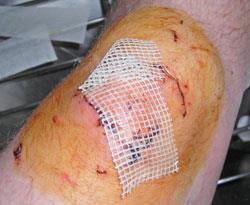
Its successor, Povidone, is a stable complex of polyvinylpyrrolidone and elemental iodine. This releases its iodine very slowly, reducing skin irritation (and stinging!). It is used in pre- and post-operative skin cleansing, for the treatment and prevention of infection in wounds, ulcers, cuts and burns; and for some eye and gynaecological infections. However, its benefits are still debated.7
To treat goitre
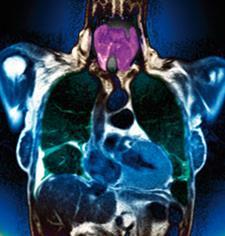
Goitre is a swelling of the thyroid gland in the neck. It can become very large and rarely causes harm unless it is so big that it obstructs swallowing or breathing, but can be unsightly. It was more common in the Midlands than the coastal regions of the UK and was formerly known as 'Derbyshire neck'.8 The traditional treatment, from the time of Galen (132-200 AD) until well into the 19th century, was to feed the patient calcined sponges, ground to a powder. Writing in 1834, the physician-chemist William Prout recollected that he had used iodine to treat the disease only three years after its discovery was announced. However, most of the credit for using iodine and its compounds to treat goitre goes to French physician Jean-François Coindet, who independently used iodine at around the same time. Unlike Prout, he promptly published his findings (in 1820), which changed practice across the western world:
Of the preparations of iodine, that of the hydriodate of potash (KI), with a superabundance of iodine is the most manageable, and the one that produces fewest accidents. For its preparation 36 grains (2.3 g) of the KI and 10 grains (0.65 g) of iodine are dissolved in an ounce (30 cm3) of distilled water. From 6 to 10 drops are prescribed at first, three times a day.9
Coindet was aware of the dangers of over-dosing and emphasised the importance of monitoring the patient during treatment. In countries where this form of goitre was once endemic, iodizing table salt has relegated this disease to history. The salt contains up to 77 ppm KI, and is presently used extensively in the USA, Nepal, New Zealand and Australia.
Iodine and syphilis
The venereal disease syphilis was very common until Ehrlich's discovery of Salvarsan , first marketed in 1910. The disease causes many symptoms, including rashes, swellings and difficult-to-heal ulcers. When it affects the brain, it causes dementia, paralysis and death. It progresses through stages, but sometimes pauses in its progression. This could give a misleading impression of therapeutic success. Many remedies became popular, with mercury (applied externally as an ointment, or internally as its salts) being the most favoured.10 It is not surprising that iodine was used in combination with mercury to treat the disease. In 1826 'proto-iodure (Hg2 I2) et le deuto-iodure de mercure (HgI2)' were being prescribed for the 'scrofulitic' complications of syphilis. However it was potassium iodide that seemed of more benefit. Although it neither rid the body of the spirochetes that cause the disease nor achieved a 'cure', it had some effect on the distressing syphilitic ulcers and pustules. In 1848 we read:
Multiplied experiments establish that the preparations of iodine possess valuable properties in the treatment of syphilitic diseases. The iodide of potassium is to be preferred to all other preparations. It is more particularly applicable to the pustular form of the disease (and) is specific in the tertiary forms ... This medicine supplies a want which was long felt by the profession (but) it is doubtful whether it is advisable to combine the iodine and mercury treatment.10
Although Salvarsan seemed to have a curative effect, particularly on the early stages of the disease, potassium iodide was still being used as late as 1935 against the 'tertiary lesions of syphilis'.
Treatment of the disease was revolutionised by the introduction of penicillin in the mid-1940s. This killed the spirochetes, curing all stages of the condition (although without reversing the significant damage caused to the body during the illness). Nevertheless, venereologists were initially reluctant to give up using KI as an adjunct therapy. Whilst acknowledging the effectiveness of penicillin and 'the arsenicals', in 1947 Dr Walsh MacDermott of New York claimed that potassium iodide was still of value in 'relieving the pain caused by diminished coronary arterial circulation secondary to syphilitic atresia of the coronary orifices'.
The pressures of warfare often drive technological advances. This was not so with iodine. While the urgent need for gunpowder led to kelp being used instead of wood ash, it was work by respected English and French chemists which led to its discovery and identification as a new element. It was the rapid transmission of this information in the scientific literature that led to its early effective medical use.
Another triumph for thoughtful observation, good science, and effective communication?
Modern concerns
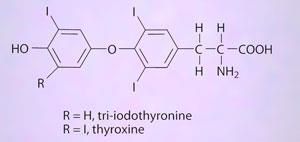
We cannot forget that iodine is one of the body's essential elements and a constituent of the hormones thyroxine (a tetra-iodo compound) and tri-iodothyronine. These regulate the rate of metabolism, affect growth, and influence many other body systems. They are produced in the thyroid, one of the largest endocrine glands in the body.
Iodine is absorbed as the iodide ion from foods such as fish, milk and table salt (to which iodine is routinely added) and is preferentially absorbed into the thyroid gland.
However, radioactive iodide/iodine I-131 is one of many products of nuclear fission, and accidents such as those involving the reactors at Fukushima, Japan can release this material into the environment:
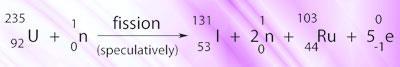
I-131 is also absorbed into the thryoid, where the β-rays it emits can damage cellular DNA causing mutations, which sometimes leads to cancer. The developing thyroid of children is thought to be more sensitive than those of adults.
Potassium iodide tablets, given in some 700-fold usual dietary amounts, can reduce this risk by saturating all the absorptive capacity of the thyroid. If the individual is then exposed to I-131, very little of this can be absorbed and it is rapidly excreted, protecting against the risk of later thyroid cancer.
Alan Dronsfield is emeritus professor of the history of science at the University of Derby. Pat Swain is a retired research scientist and school teacher. Pete Ellis is professor of psychological medicine at the medicine University of Otago, Wellington, New Zealand.
Further reading
-
Pat Swain has written more about Bernard Courtois and the discovery of iodine.11
-
Some of the medical references are dealt with in the article by Rosenfeld.12
-
Patrick Walter of Chemistry World has also written a blog article about Bernard Courtois and a case of mistaken identity. (see reference 13)
References
- B Jones, Faraday's Life and Letters, Vol 1. London: Longmans, 1870
- J L Gay-Lussac, Ann. Chim., 1813,88, 311
- H Davy, Ann. Chim., 1813, 88, 322
- H Davy, Phil. Trans. R. Soc. Lond., 1814, 101, 74
- L-G Toraude, Bernard Courtois et la D?couverte de l'Iode. Paris: Vigot Freres, 1921
- Education in Chemistry, September 2009, p153
- D Norman, Br. J. Nurs., 2003, 12(6), S30
- http://en.wikipedia.org/wiki/Goitre - (This link will open in a new window)
- J Eberle and J Webster (Eds), The American Medical Recorder, 1822, 5, 362.
- Education in Chemistry, November 2004, p162
- P Swain, Bull. Hist. Chem., 2005, 30(2), 130(http://bit.ly/kGWWgt (PDF) - (This link will open in a new window)
- L Rosenfeld, J. Chem. Ed., 2000,77, 984 (DOI: 10.1021/ed077p984 - This link will open in a new window)
- http://bit.ly/iNOzzC - (This link wil open in a new window)






No comments yet