Loss of bone tissue with age - osteoporosis - is responsible for around two million hip fractures per year. While metal-based replacements are the favoured treatment, these have a limited lifespan of 15 years. 'Bioactive' ceramic and glass alternatives could improve the quality of life for millions of people.
-
Certain glasses and ceramic materials bond to living bone
- Implants are now being designed not only to replace damaged bone but also to repair and regenerate defective tissue in vivo
Degenerative bone disease is a growing problem throughout the world. Approximately 90 per cent of the population over the age of 40 suffer to some extent with this condition. While normal repair processes fail with age, injury, infection or excessive loading may also be contributory factors. Moreover, life expectancy in prosperous parts of the world currently stands at 80+ years, which means that many people will exceed the natural lifespan of their own connective tissues, particularly bone and cartilage. Current statistics reveal that 33 per cent of women and 17 per cent of men, between the ages of 80 to 90, get hip fractures.
A general problem with artificial replacement joints - usually a polished metal ball mounted on a metal femoral stem and a polymer or alumina cup - is that of stress transfer from synthetic materials, whose mechanical moduli (elasticity) are very different from that of the host bone. Changes in mechanical loading following hip replacement cause demineralisation of bone from the inner wall of the femur, which can lead to loosening of the femoral stem. Additionally, wear debris from the articulating prosthetic joint is also a common problem that provokes the body's immune response and tends to require revision surgery. While bone transplantation is an alternative, the lack of donors and tissue compatibility limit the usefulness of this approach. Current research is focusing on the design of bioactive glass and ceramic scaffolds that could be used to enhance the body's own repair mechanisms, and thus regenerate compromised bone tissue in situ.
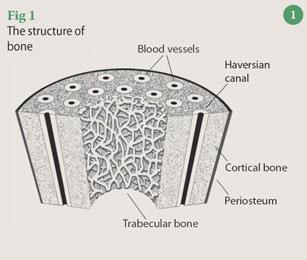
Bone structure
Broadly speaking, bone comprises a network of collagen fibres that are impregnated with crystals of hydroxyapatite, Ca5(PO4)3(OH), and some calcium carbonate. Collagen is a flexible, tough protein that dictates the shape of the bone, to which the hydroxyapatite phase is bonded via polar functional groups on the protein molecules and provides additional strength. Enclosed within this solid collagen-mineral matrix are channels carrying blood vessels, nerves and lymphatics (Fig 1). Long bones, such as the femur, comprise an exterior, relatively rigid, dense 'cortical' bone with a compressive strength of 100-230 MPa and a Young's modulus of 7-30 GPa. The interior of this bone consists of a spongy, honeycomb-like structure composed of the collagen-mineral matrix filled with soft marrow, fat and bone cells. This porous 'trabecular' bone is comparatively weak and more flexible than cortical bone, having a compressive strength and Young's modulus of 2-12 MPa and 0.05 -0.5 GPa, respectively. The specific arrangement of cortical and trabecular bone, and the difference between their mechanical properties provides a smooth stress gradient from tendon to bone and mediates the transfer of forces along the bone.

The structure and function of bone is maintained by a balance between the activities of bone-forming cells - osteoblasts - and the bone resorbing cells - osteoclasts. A reduction in the normal stress patterns exerted on bone causes demineralisation (also known as bone atrophy) during which osteoblast activity diminishes, causing a net resorption of hydroxyapatite and calcium carbonate by the body. Bedridden patients, sedentary elderly people and astronauts are particularly prone to reduced mechanical stress on their bones and the consequential loss of bone density. The effects are particularly severe in trabecular bone and greatly reduce its mechanical strength and resistance to fracture. The trabecular bone is also vulnerable to demineralisation with ageing. In a study of the human femur, tensile strength was found to decrease from around 120 MPa to 65 MPa from the ages of 20 to 95 years.
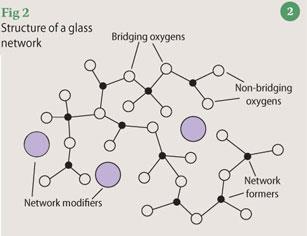
Introducing ceramics and glasses
Ceramics are inorganic solids held together by both covalent and ionic bonds, and are composed of metallic or semi-metallic elements in combination with oxygen, nitrogen, silicon or carbon. Many are based on silicates, aluminosilicates, borates and zirconates and are characteristically hard, durable, chemically inert and heat-resistant. Inorganic glasses are a distinct category of non-crystalline ceramics, whereas 'glass-ceramics' comprise crystalline phases embedded in an amorphous matrix.
Ceramics currently used in clinical applications - bioceramics - are classified according to the way they interact with living tissue:
-
virtually inert ceramics show little or no biological response when placed in the body. Materials of this type include alumina (Al2 O3) and zirconia (ZrO2) and are, for example, used as femoral heads in artificial hip joints;
-
porous ceramics, such as synthetic hydroxyapatite, have large surface areas and become fixed by biological ingrowth into their pores. They are coated onto metal stems, for example, for femoral components of artificial hips;
-
bioactive materials, such as calcium silicate-based glasses, ceramics and glass-ceramic composites chemically bond with living tissue. They are currently used to fill defects in the jaw following the removal of teeth and as replacement for ossicles in the middle ear to restore hearing;
-
resorbable ceramics, eg calcium phosphate cements, are soluble under conditions prevailing within the body, and are generally removed by gradual dissolution. They are used, for example, to repair bone defects during craniofacial reconstruction and dentistry.
A new generation of bioactive glasses
The discovery that certain glass and ceramics could bond with living bone suggested that, rather than strive for inertness, implant materials might be tailored to elicit a positive response from the host tissue. Scientists hypothesised that bioactive implants might also be designed to aid the repair of damaged or defective tissue by naturally degrading at an equivalent rate to that of the tissue regeneration. Subsequent research has revealed that particular formulations are also capable of bonding to soft tissues such as skin. Thus, in addition to their use in the repair of defective tissue in vivo, bioactive substrates could also be used to enhance the performance of tissue scaffolds used to support artificially engineered tissue cultures in vitro.
Bonding to bone was first demonstrated for a four-component, conventional, melt-derived glass - SiO2 -Na2 O-CaO-P2 O5. The approximate weight proportions required for bioactivity are ca 30-55 per cent SiO2, 20-25 per cent Na2 O, 15-30 per cent CaO and 4-7 per cent P2 O5. These bioactive glasses differ little from traditional soda-lime-silica glasses, which are used for containers and windows, and which contain a quantity of P2 O5, less than 60 per cent SiO2, and higher proportions of Na2 O and CaO.
Melt-derived glasses are prepared by rapid cooling from the liquid state so that crystallisation does not occur and the material becomes rigid by virtue of its increased viscosity. Such glasses are composed of an interconnected near-random distribution of covalently-bonded 'network formers', for example silicate chains, whose negative charges are balanced by coordination with Group I and/or II cations or 'network modifiers' (Fig 2).
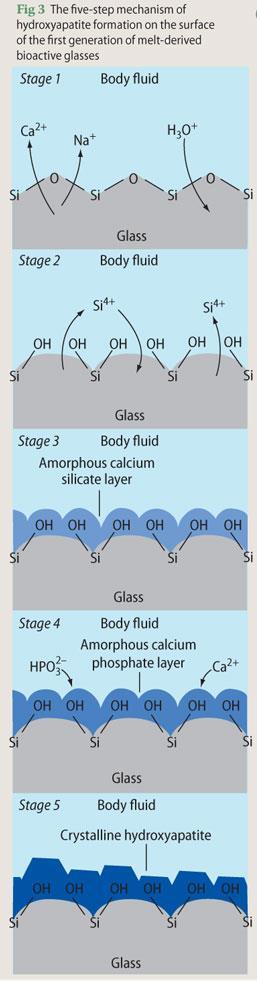
The appropriate oxide constituents of the bioactive glass mixture (such as silica, alumina, calcium oxide, calcium phosphate etc) are heated to around 1300°C for a sufficient length of time to homogenise the mixture. During this process, the giant three dimensional lattice structures of the network formers (in this case silica, SiO2) break down and the network modifying cations (such as Ca2+ and Na+) become associated with the non-bridging oxygen atoms. The liquid is then rapidly cooled, often by casting into the required shape. The resulting glass article will contain 'frozen-in' stresses and will also comprise between 10 and 40 per cent residual porosity, both of which can be removed by annealing at approximately 500°C.
More recently, bioactive glasses have been prepared by the sol-gel process. This involves the pH-controlled formation of a three dimensional porous gel matrix from a colloidal suspension (a sol). The gel can then be matured, dried and thickened into a glass by heating. The gel can be obtained by the dispersion of colloidal powders or by the controlled hydrolysis and condensation of liquid metal alkoxide precursors under acidic or basic conditions. Most bioactive glasses, however, contain calcium and cannot be prepared under basic conditions which would cause the precipitation of insoluble calcium salts.
One advantage of the sol-gel process over that of conventional glass-making methods is the possibility of producing porous glasses having carefully controlled textural parameters such as pore structures, high surface areas and low densities.
The bone-glass interaction
To bond to bone, a hydroxyapatite layer must form on the surface of the implanted material. This is similar in composition and structure to the apatite phase present in bone and having formed, it provides a focus for the deposition of collagen fibrils and for the attachment of stem cells. These cells then differentiate into osteoblasts and initiate the natural bone-building process.
Under normal physiological conditions, our body fluid is highly saturated with the components of hydroxyapatite so, once apatite nuclei are formed, they will grow spontaneously. Additionally, the dissolution of ions, such as Ca2+ and PO43-, from the glass will increase the degree of saturation of body fluid and enhance the kinetics of hydroxyapatite precipitation. The dissolution of Group I and II network modifying cations from the glass also raises the local pH, which in turn reduces the solubility of hydroxyapatite and thus assists the precipitation process.
The mechanism of hydroxyapatite formation on the surface of the first generation of melt-derived bioactive glasses is described in five steps (Fig 3):
-
Rapid diffusion-controlled ion exchange of network modifying Ca2+ and Na+ ions from the glass with H3 O+ ions from the body fluid. This step increases the pH at the implant-bone interface.
-
Development of silanol groups (Si-OH) at the surface via the partial dissolution of silica at the interface.
-
The condensation and repolymerisation of the silica-rich surface layer to form an amorphous calcium silicate layer.
-
Incorporation of HPO42- and Ca2+ ions from the body fluid into the calcium silicate layer to form an amorphous calcium phosphate layer.
-
Crystallisation of the amorphous calcium phosphate layer into biologically equivalent hydroxyapatite.
Porous sol-gel-derived glasses exhibit bone-bonding interactions over a significantly larger compositional range than that of traditional melt-derived glasses. Even pure SiO2 gels prepared by this method have been found to be bioactive. This is because the high surface areas and residual Si-OH groups of the porous gels promote the dissolution, condensation and repolymerisation processes of steps two and three of the hydroxyapatite formation mechanism. The subsequent development of the amorphous calcium silicate layer and incorporation of Ca2+ and HPO42- ions can then take place. Conversely, the low surface area of non-porous, melt-derived SiO2 and absence of Si-OH groups limit the rate of dissolution of this material in the physiological environment to such an extent that the layer of hydroxyapatite does not develop. It is for this reason that melt-derived glasses having compositions of greater than 60 mol per cent SiO2 do not generally exhibit bioactivity.
Testing for bioactivity
In 1990 a team of Japanese researchers at Kyoto University devised a pre-clinical in vitro laboratory test which reproduces the in vivo surface structural changes that take place when ceramics and glasses are implanted in the body. The test involves immersing the candidate bioceramic in a 'simulated body fluid', consisting of a variety of salts, whose ionic composition approximates that of human body fluid (Table 1). Typical immersion periods range from 30 minutes to seven days at 37°C. Infrared spectroscopy and/or electron microscopy are then used to determine whether a layer of hydroxyapatite has precipitated onto the surface and also the fluid composition can be monitored over time to determine the kinetics.
| Table 1 - Ionic compositions or human and simulated body fluids | ||
|---|---|---|
| Species | Human blood plasma/mM | Simulated body fluid/mM |
| HPO2-4 | 1.0 | 1.0 |
| Na+ | 142.0 | 142.0 |
| K+ | 5.0 | 5.0 |
| Mg2+ | 1.5 | 1.5 |
| Ca2+ | 2.5 | 2.5 |
| Cl- | 103.0 | 147.8 |
| HCO-3 | 27.0 | 4.2 |
| SO2-4 | 0.5 | 0.5 |
| pH | 7.2 | 7.2 |
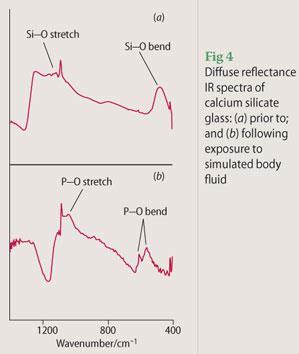
Figure 4 shows the diffuse reflectance infrared spectra of a sol-gel-derived calcium silicate glass prior to and following immersion for seven days in simulated body fluid. During diffuse reflectance IR, the light reflected from the surface of the sample is detected rather than that which passes through the sample as is the case for transmission or absorption IR spectroscopy. Prior to immersion in simulated body fluid, Si-O stretching modes are observed at 1100 and 1250 cm-1 and Si-O bending modes are noted at 500 cm-1. Following immersion, the reflectance spectrum is dominated by signals from the precipitated hydroxyapatite: P-O stretching and bending modes are observed around 1120 and 650 cm-1, respectively. Thus, this calcium silicate glass exhibits in vitro bioactivity so the next stage in pre-clinical trials would be to investigate its ability to sustain an osteoblast cell culture.
Clinical applications and current research
Bioactive glasses can be used surgically as solid articles, granules, powders and very fine particles that can be injected. As well as replacements for ossicle bones in the middle ear, bioactive glass is also used in oral surgery to fill defects in the jaw. When teeth are extracted, for example, the associated changes in the mechanical loading pattern cause bone resorption and this can, in part, be mitigated by bioactive glass implants. Bioactive glass particles have also been mixed with autogenous bone in the reconstruction of the lower jaw following tumour removal and trauma surgery.
Exciting new research has shown that the dissolution products of bioactive glasses, particularly silicon species, operate at a genetic level to influence the osteoblast cell cycle. The presence of bioactive glasses was found to enhance the expression of genes and increase the production of growth factors which regulate bone growth. On the basis of this discovery, scientists are now investigating the impact of incorporating silicon into other implantable materials such as synthetic hydroxyapatite. Results so far have shown that in vivo bone regeneration is enhanced for implanted silicon-substituted hydroxyapatite granules compared with that of its unsubstituted counterpart.
There is also a great deal of research interest in the use of bioactive materials for tissue scaffolds that mimic the structure of trabecular bone. Tissue scaffolds are three dimensional matrices which are used to template and support the growth of bioartifical tissues in vitro. The challenge is to prepare resorbable scaffolds of suitable geometry and bioactivity to support the growth of artificially-seeded tissues which can be tailored to fit specific bone defects. Research is currently being done to fine-tune the architecture and resorption characteristics of sol-gel-derived bioactive glasses. Foaming agents and surfactants are being incorporated into the sol-gel reaction mixture to introduce interconnected pores which emulate the porous structure of trabecular bone.
These inorganic materials that can augment the body's own ability to regenerate are set to become significant in future clinical approaches to restore function in damaged tissue. The possibility of producing bespoke engineered
tissues seeded from the patient's own cells to minimise rejection is an innovative alternative to the problems currently associated with prosthetic implants and with donor organs. If successful, it will dramatically improve the quality of life for millions of people worldwide.
Dr Nichola J. Coleman is a lecturer in chemistry in the school of science at the University of Greenwich, Medway Campus, Chatham Maritime, Chatham, Kent ME14 4TB; John W. Nicholson is professor of biomaterials chemistry at the same institution.
References
J. W. Nicholson, The chemistry of medical and dental materials. London: RSC Materials Monograph, 2002.
L. L. Hench and J. Wilson (eds), An introduction to bioceramics. Singapore: World Scientific Publishing, 1993.
J. R. Jones and L. L. Hench, Current Opin. Solid State and Mat. Sci., 2003, 7, 301.






No comments yet