Josh Howgego finds out how converting radioactive waste into novel glass materials could be a safe, secure way to deal with the legacy of nuclear power stations

There aren't many people suffering from radiation damage in the UK, so at first sight it might seem like there's not much research left to do when it comes to nuclear waste and no need to invent new ways of dealing with it.
However, that impression is quite wrong, according to Neil Hyatt, a professor of nuclear materials chemistry at the
University of Sheffield, UK. Much of the UK's high level nuclear waste is stored at Sellafield, Cumbria, in a high-security facility. But it's only there temporarily. Dealing with nuclear waste is difficult, so it is often put into interim storage until industries, government and the local residents can agree on where it should go. Eventually the waste will be buried underground.
It's not just spent nuclear fuel that needs to be disposed of either. In the UK ageing nuclear facilities - some over 40 years old - need to be safely decommissioned and their stored wastes safely treated. In November 2012 the National Audit Office published a report saying that some older components at Sellafield had deteriorated so much they posed a 'significant risk to people and the environment'. Working out how to safely dispose of all the various pieces of the plants - from old pipes to thermal cladding - is time-consuming, says Hyatt. Until recently, consecutive nuclear reactors were built using variable designs. That means thought has to go into how to decommission each reactor or facility separately. On top of that, Hyatt says the regulations on how we deal with radioactive waste are getting tougher all the time.
Scientists must also think far into the future when dealing with nuclear waste, because that's how long it takes to decay. Working out the exact timescale is more complicated than you might think, since different isotopes of radioactive elements decay at different rates. For some idea of the situation, it's worth thinking of the two most common elements in nuclear fuels, plutonium (239Pu) and uranium (238U), which have half-lives of 2.41 x 104 years and 4.46 x 109 years respectively. (See Radioactive decay). So, scientists need to make sure that waste products are stored securely for millions of years. As Hyatt explains: 'Nuclear waste is pretty nefarious stuff. It's an intergenerational issue, so we need to take responsibility for it.'
A nice glass of plutonium

At the moment most nuclear waste is mixed into cement before being transferred to drums that can be stored deep underground. But Hyatt thinks a much better way to encase the waste would be to 'vitrify' it; that is, turn it into a piece of glass. In such a state the radionuclides are safely held in a solid solution of other elements that quench the radiation.
The main problem with cementation, says Hyatt, is that it is indiscriminate: pretty much all the waste materials are mixed up, and stored without much chemical effort being expended to separate out what is radioactive and what is not. That means more material ends up being stored than is strictly necessary.
Nuclear facilities do, however, already use what's called the Purex process to recycle the plutonium and uranium remaining in their used fuel. The process involves chemical separations based on the solubility of the different elements. These are simple in theory, but do present an engineering challenge: the end result delivers pure plutonium and uranium (which can be re-used), plus thousands of litres of highly radioactive waste as a solution in nitric acid (HNO3). This unpalatable material, says Hyatt, is where most of the UK's high-level nuclear waste originates. From here, the nitric acid solution can be mixed with simple chemicals, like sodium carbonate and silicon dioxide (sand), and heated to form a molten glass that cools to form highly durable glassy solids that trap the waste.
According to Hyatt there are several reasons why these glassy materials are the nuclear waste encapsulating materials we should opt to use in future. Because the radioactive nuclides are locked into a chemical structure, they are packed into a smaller space than if they were simply mixed with cement mechanically and so, require less storage space. Another advantage, says Hyatt, is the transformation of the liquid waste into something solid. 'Obviously having a solid is better than having a liquid, which could leak out of a barrel - we wouldn't want to be storing massive quantities of [liquid],' says Hyatt. 'But on top of that the materials are mechanically robust, and, we hope, resistant to chemical degradation, to dissolution by groundwater and to radiation damage.'
Radioactive decay
Radioactive decay is distinct from the sort of thing that happens when you leave a cheese sandwich on your desk for too long. Instead, it refers to when atoms eject parts of themselves spontaneously. Most common atoms are quite 'happy' with their quota of subatomic particles, but heavier atoms, especially the actinides (elements 89-102) have so many protons and neutrons that they become unstable, and spew out bits of themselves at unpredictable intervals.
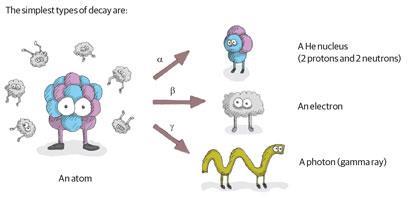
Although it's impossible to tell when individual atoms will decay, on a statistical level the process is predictable: '50% of the atoms will decay in 70 years,' say. Hence the measure of reactivity in half-lives: the time it takes for half
the atoms to decay.
But why limit the glass forming chemicals to sodium carbonate and silicon dioxide? Hyatt says the great thing about vitrification is that there is no hard and fast rule on what the glass forming chemicals should be. 'What I'm interested in developing is a toolbox of different glass compositions, so that we can call on different glass or ceramic material tools to deal with different types of waste,' he says (see table 1). 'There is no catch-all, magic bullet for this.'

One good place that scientists could look at to establish what makes a good material to encase actinide elements is their natural ores. Plutonium doesn't occur naturally, of course, since it's too radioactive. But uranium (and other actinide) ores have sat securely in the ground for many millions of years. 'These minerals are some of the oldest known on the earth. And they have retained their actinides - uranium and thorium - under conditions of weathering for millions, or hundreds of millions of years,' says Hyatt, 'That's in excess of the timescales that we need to demonstrate safe disposal of radioactive waste.' Hyatt uses the structure of these natural minerals as his starting point to make synthetic ceramic copies in his laboratory.
Some people occasionally worry that Hyatt might have a conflict of interest in his work. But these natural materials show that it is possible to encapsulate radioactive elements in minerals for geological timescales. 'No one could have tampered with these minerals,' says Hyatt. 'So it is a uniquely powerful piece of evidence, and it also helps us show the public that what we're doing really is safe.' (See Box - Nuclear on the airwaves: a debate of extremes?).
Simulating millennia
Over time these crystal structures will change though. Once in a while, one of the stored actinide nuclei will emit an alpha particle, which will go shooting off and smash into other atoms in the crystal lattice. Those impacts add up, and mean that over long periods of time the atoms in the crystal move around, potentially ruining the integrity of the material. Not only that, but as the eons pass the buried waste materials will likely be flooded - water tables can change a lot in a million years - and the effects of the salty, mineralised water on the waste need to be accounted for. Could this liquid react with the material and lead to a rupture that would release dangerous radioactive material?
To check out these possibilities Hyatt speeds up the decay process of the actinides, simulating several millennia's worth of radioactive decay in a few minutes. To do this, he uses fast ion beams from a particle accelerator to irradiate his glass and ceramic materials. Instead of using dangerous radioactive elements, Hyatt uses model structures that contain atoms like cerium, which has similar chemistry to plutonium. The collision of the ions with atoms in the materials causes what Hyatt calls 'atomic recoiling' and mimics what would happen when a plutonium or uranium atom releases an alpha particle. In this way, Hyatt and his colleagues can study the effects of long-term radiation on their materials.
In designing ceramic materials to incorporate actinides, Hyatt says it's 'generally best to play safe and avoid any chance of initiating a nuclear chain reaction.' To try and do that, he and his team can experiment with adding in a sprinkling of elements that are particularly good at absorbing radiation like hafnium and gadolinium. Of course, small changes can potentially make unexpected changes to the material's properties, so each new structure needs to be assessed carefully.
Creator or destroyer?

Global society has had a kind of love-hate relationship with nuclear power over the years, and that can be easily demonstrated by the changing status of nuclear power in Japan. Back in 2011 the country was ravaged by the Fukushima disaster, and subsequently shut all of its 50 nuclear power stations. However, two have recently been reopened and there are signs that the Japanese may not be able to do without nuclear power, which until the disaster, supplied a third of the country's energy. In the sleepy few days between Christmas 2012 and the start of 2013 the newly-elected Japanese government announced they would be reviewing the previous administration's decision to phase out nuclear power by 2040.1
In the UK too, nuclear technology will have a place in the future of energy generation. In October 2012, the government revealed that 16 GW of new nuclear power capacity will be built in the UK by 2025. They say this will create 33 000 new jobs.
Marcel LaFolette, a research associate at the Smithsonian Museum, US, has studied how American TV depicted science from 1948 to 1990. One theme she identified was the packaging of science as what she called a 'creator/destroyer.' Science has the power to heal sick people and send men to the moon but it can also bring trouble. Nuclear power was, for LaFolette, a paragon of her 'creator/destroyer' science.
We desire the cheap, clean energy nuclear power brings us. But, inevitably, we must also stomach the responsibility of safely storing the waste products.
Nuclear on the airwaves: a debate of extremes?
The Hitchhiker's Guide to Nuclear is a podcast about all things nuclear. It's run by two PhD students who study nuclear materials in the Nuclear First PhD training centre that Hyatt directs. The podcast2 aims to be engaging in style while also being educational, explains Matt Gunther, one of the hosts. He wants to enable people to make informed opinions on nuclear technology.
'Last month we discussed the nuclear waste situation in Cumbria,' says Gunther, speaking in December 2012. 'Then we reported on a US nuclear submarine commander faking his own death to fool his mistress.'
Gunther says he believes most talk of nuclear power in the UK is a 'debate of extremes' and hopes the podcast 'will generate some sort of middle ground.' It's hard to tell how effective the project has been, but Gunther cites a 100-strong subscriber list on iTunes and 350 followers on Twitter.
Hyatt agrees with Gunther. Since he is partly funded by the public, he says he must communicate his findings with them. It's about 'equipping people to make informed decisions,' he says. 'I'm not necessarily a champion for nuclear, but I think, at the moment, there is a strong case for saying nuclear should be part of how we generate energy in this country.'
Josh Howgego is a science writer based in London, UK






No comments yet