Triazole synthesis provides an excellent example of a reaction that has relevance. These chemicals have important applications in the chemical and pharmaceutical industries, and have the potential to illustrate principles of green chemistry to undergraduates.
-
Industrially-relevant syntheses provide 'discovery-based' approach to lab work
-
No solvents, no waste, and no toxic metal catalysts - and done in a microwave oven in minutes

Synthetic 1,2,3-triazoles, with their unusual N,N, bonding arrangements, have become important organic substrates owing to their many possible applications, ranging from industrial uses to a variety of pharmaceutical applications. For example, triazoles are used in the manufacture of dyes and inks, corrosion inhibitors, and polymers. Additionally, triazole-containing molecules have been shown to have anti-allergenic,2 anti-bacterial and anti-HIV3 activities, and are under study as drugs for the treatment of osteoarthritis4 and obesity.5 These heterocycle compounds are particularly interesting for pharmaceutical applications because they are more likely to be water soluble than standard aromatic systems, and are stable under biological conditions.
A green synthesis
As part of our ongoing efforts to acquaint undergraduates with the latest and most significant advances in synthetic chemistry we have developed a series of experiments that focus on environmentally friendly or 'green' methods.7 Specifically we exploit two 'green' principles, ie preventing waste is better than cleaning it up; and less toxic alternatives should be used whenever possible. Consequently, the reactions are done in the absence of solvents (thus eliminating the major source of waste in any lab experiment) and without added catalysts, since many catalysts are toxic, metal-containing complexes. The experiments are done in a domestic microwave oven, which offers several advantages over standard heating methods.8 Not only are microwave-assisted reactions faster than their classical counterparts, they are generally cleaner, require less energy to run, and allow for easy heating of small-scale reaction mixtures.
Traditional versus green syntheses
The synthesis of 1,2,3-triazoles involves the 1,3-dipolar cycloaddition of an alkyl azide to an alkyne (see equation (i)). Theoretically this addition results in perfect 'atom economy' for this reaction, ie every atom of starting material is incorporated into the product molecule.9

Traditional approaches to the synthesis of these molecules have limitations which make them less suitable for use in the undergraduate lab. For example, one classical approach involves doing the reaction over many hours and under extremely high pressures inside a steel bomb reactor,10 which would be unsafe for new undergraduates. Another widely used approach requires the use of a mixed solvent system and a copper catalyst.11 Although both these methods provide very high yields of products, they fail to achieve the principles of green chemistry we want to emphasise.
A green method

Our method involves mixing an organic azide and an alkyne in an Erlenmeyer flask, covering with a watch glass, and heating in a domestic microwave oven (we use a 900 W Emerson model 8987B). Reaction times can vary from 10 s to 15 minutes (depending upon the reagents chosen), with progress being followed by TLC. The 1,2,3-triazoles are produced in good to excellent yields (46-99 per cent) from a variety of different alkynes and azides.12
Safety
Microwave ovens should always be run with caution, and care should be taken to avoid overheating the reactions. Since the reagents are volatile, never run a reaction in a sealed vessel because pressure build-up could lead to an explosion. It is always best to place the microwave oven inside a ventilated hood wherever possible, and to keep reaction scales small (1-3 g) to prevent any potential hazards.
Experiment variations and learning outcomes
Our students are chemistry majors who have demonstrated competency in all of the basic laboratory techniques - extraction, recrystallisation, distillation, chromatography etc. Our objectives are to stress the application of these techniques in reactions which have relevance in the current literature, and get students to analyse spectroscopic data to determine reaction outcomes.

To accomplish these goals we employ a 'discovery-based' approach. Thus the experiments are presented to the students as reaction procedures in which the outcome of the reaction is left for the student to determine. Students are given general instructions on how to do the reaction, but are left to design their own purification procedure (with guidance). Since they are not told the outcome of the reaction, they rely on their analysis of spectroscopic data to uncover the product's structure.
We begin by discussing green chemistry and its applications, focusing on what it means to be 'green' and how this can be accomplished in the laboratory. We also discuss microwave-based synthesis and its associated advantages and disadvantages. Industrial-scale microwave synthesis is not yet common, but you could encourage your students to think about the size of the microwave oven that would be required to make a tonne of the same compound. This could lead to a discussion of microwave power and how it drops with distance from the source, necessitating flow through systems in place of batch reactors.
Students are then sent to the laboratory to do their experiments and to determine their product(s). They present their results in the form of a written paper which is formatted as a journal article containing background, results, discussion, and experimental sections. We ask that the background section reflects an analysis of the current literature procedures which are used in the synthesis of their compounds and that the students discuss the concept of green chemistry as it applies to the literature methods and to the reaction they have just completed.
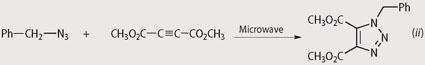
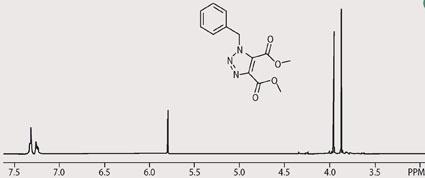
The triazole synthesis has the advantage of being easily tailored to meet the needs of students at different levels of understanding, depending upon which starting materials you provide. For example, if students have basic analytical skills you can choose a straightforward synthesis using an symmetrically substituted alkyne, which results in a single product in high yield. The reaction of benzyl azide with dimethyl acetylenedicarboxylate is an example, which gives the product in 98 per cent yield after 30 s at 30 per cent power (see equation (ii)). The crude reaction product in this example is clean enough to analyse without further purification. Students may study this product by infrared spectroscopy (IR), mass spectrometry (MS) or nuclear magnetic resonance spectroscopy (NMR). As an example, the 1H-NMR of dimethyl 1-benzyl-1,2,3-triazole-4,5-dicarboxylate is shown in Fig 1.
For more experienced students, the experimental difficulty can be increased by using a starting material which will produce two isomeric products. This is accomplished by using an asymmetrically substituted alkyne, such as phenylethyne (see equation (iii)). In this reaction, both 1-benzyl-4-phenyl-1,2,3-triazole and 1-benzyl-5-phenyl-1,2,3-triazole are produced in 48 per cent overall yield after nine minutes of microwave heating at 80 per cent power. The formation of isomeric products adds an extra separation step to the procedure, which is effectively accomplished by flash chromatography on silica gel. In this case analysis by IR and MS are helpful, but the 1H-NMR is needed to distinguish between the isomers, either by literature reference or by simple NOE (nuclear Overhouser effect) difference experiments.

For advanced students, you can bring even more challenge to the experiment. Our studies have shown that an unexpected decarboxylation reaction takes place whenever propiolic acid derivatives are used in these triazole syntheses. Thus the reaction of benzyl azide with phenylpropiolic acid results in the same two product isomers found in the reaction of benzyl azide with phenylethyne, in a 46 per cent overall yield after only six minutes of microwave heating at 30 per cent power (see equation (iv)). Once again, two isomeric products are formed in this experiment, so a chromatographic separation is necessary. However, the unexpected decarboxylation reaction adds another layer of complexity to the analysis. In fact, the decarboxylation was not immediately obvious to our students from the 1H-NMR spectrum alone, so 13C-NMR, IR and/or MS analyses were also done to solve the products' structures.

And finally...
By presenting this reaction in a discovery-based approach, emphasis is placed on students' understanding of the techniques used in purification and on the importance of analytical skills for the determination of product structure. As teachers you have the flexibility to vary the difficulty and learning objectives of this experiment to match the skill levels of your students by simply changing the starting materials.
Student feedback suggests they enjoy doing these microwave-based experiments, and are particularly pleased with the speed with which they can be done. Even more importantly, they will often comment on or complain about subsequent experiments if they feel the reactions do not live up to the goals of 'green', environmentally friendly chemistry, which suggests they are using their new-found skills towards the analysis of other reactions.
Dr Cynthia Kellen-Yuen is assistant professor of chemistry in the department of chemistry at the California State University, 6000 J Street, Sacramento CA 95819-6057; Cathleen Roush is a MS student in the same department.
References
- M. J. Genin et al, J. Med. Chem., 2000, 43, 953.
- D. R. Buckle et al, J. Med. Chem.,1986, 29, 2262.
- R. Alvarez et al, J. Med. Chem., 1994, 37, 4185.
- J. S. Tullis et al, Bioorg. & Med. Chem. Letts, 2003, 13, 1665.
- L. L. Brockunier et al,Bioorg. & Med. Chem. Letts, 2000, 10, 2111.
- N. Kaval et al, J. Comb. Chem., 2005, 7, 490.
- P. T. Anastas and J. C. Warner, Green chemistry: theory and practice. Oxford: OUP, 2000.
- D. Bogdal, Microwave-assisted organic synthesis, Vol 25. San Diego: Elsevier, 2005.
- K. M. Doxsee and J. E. Hutchison in Green organic chemistry. Strategies, tools, and laboratory experiments, p91. Toronto, Canada: Brooks/Cole, 2004.
- S. A. Lermontov, S. V. Shkavrov and A. N. Pushin, J. Fluorine Chem ., 2000, 105, 141.
- A. F. Joosten et al, Eur. J. Org. Chem., 2005, 2005 (15), 3182.
- C. Kellen-Yuen and C. Roush, unpublished results.






No comments yet