Nina Notman finds out how molecular motors have the potential to fuel advances in smart materials, soft robotics and molecular synthesis

Specification links
Download a list of specification points this article supports from the Education in Chemistry website: rsc.li/2wDpNrg
‘Our bodies are full of tiny nanomotors,’ explains Ben Feringa from the University of Groningen in the Netherlands. These natural molecular motors power everything inside us, from our muscles, to the transport of molecules across cell membranes, to DNA synthesis. They respond to external stimulation by performing mechanical motion, just like the macro-sized motors all around us. ‘In the macroscopic world there are motors everywhere: in our cars, our planes, our manufacturing machines and in robots,’ Ben points out.
For many years, scientists have been trying to construct molecules which function as tiny artificial nanomotors that mimic those found in biology. In 1999, Ben built the first synthetic molecular motor – a feat that earned him a third share in the 2016 Nobel prize in chemistry. A handful of artificial nanomotors, about 1000 times smaller than the width of human hair, have since been built.
Download the full text of this article (MS Word or pdf).
‘Nanoscience and its scale’ and ‘the three dimensional nature of molecules’ are two concepts that students meet in school yet often find difficult to visualise. In the article, ‘Nanomotors power on’ we are introduced to molecules about a thousand times smaller than the width of a human hair that can act as motors. Comparing their function with familiar machines such as windmills and cars helps visualise how these nano-motors work.
The article provides an excellent example of how the scientific concepts learnt at school (like the nanoscale, shapes of molecules an geometric isomers) provide a base on which even the most cutting edge scientific discoveries are built.
‘Nanoscience and its scale’ and ‘the three dimensional nature of molecules’ are two concepts that students meet in school yet often find difficult to visualise. In the article, ‘Nanomotors power on’ we are introduced to molecules about a thousand times smaller than the width of a human hair that can act as motors. Comparing their function with familiar machines such as windmills and cars helps visualise how these nano-motors work.
The article provides an excellent example of how the scientific concepts learnt at school (like the nanoscale, shapes of molecules an geometric isomers) provide a base on which even the most cutting edge scientific discoveries are built.
Seeing the light
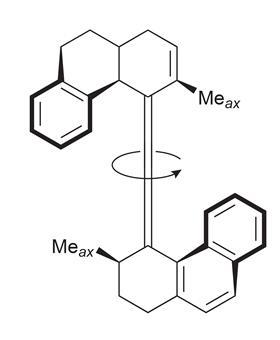
Ben’s first motor was an alkene with bulky functional groups that spun around its central C=C double bond under ultraviolet (UV) light. Controlling the direction of motion is the largest challenge with nanomotors. ‘The molecules are constantly moving through random thermal motion, Brownian motion,’ explains David Leigh, another nanomotor designer at the University of Manchester, UK. ‘What you have to do is to block all the random motion that’s occurring in the directions that you don’t want, so that the only thing that’s left is motion in the direction that you do want.’
The first molecular motor spins in a single direction thanks to its design; the ‘overcrowded’ alkene’s two large functional groups resemble propeller blades. Pulses of UV light cause the configuration of the central double bond to switch between cis and trans, and the blades to rotate 180 degrees. The ratchet-like shape of the molecule prevents reverse rotation, meaning further UV pulses keep the blades spinning in the same direction.
Naming geometric isomers, ages 16–18
The first molecular motors discussed in this article relies on restricted rotation about a carbon-carbon double bond. When the substituents on each carbon atom of the double bond are different, this results in the molecule displaying geometric isomerism.
Download this worksheet (MS Word or pdf) and answers (MS Word or pdf) that guides students through how geometric isomers are named and assesses their understanding of the topic before asking them to apply their understanding directly to molecular motors.
Naming geometric isomers, ages 16–18
The first molecular motors discussed in this article relies on restricted rotation about a carbon-carbon double bond. When the substituents on each carbon atom of the double bond are different, this results in the molecule displaying geometric isomerism.
Use the worksheet provided that guides students through how geometric isomers are named and assesses their understanding of the topic before asking them to apply their understanding directly to molecular motors. Download copies from the Education in Chemistry website (rsc.li/2wDpNrg).

New nanomotor designs developed from there. ‘The main reason for the second generation design was so that we could attach motors to surfaces and make our nano-windmill park,’ Ben says. Tiny windmills are an appropriate choice for a Dutch chemist wishing to demonstrate his technology, but it was Ben’s nanocars that really caught the media’s attention.
In 2011, the team revealed a molecular chassis with one of their signature nanomotors on each corner acting as wheels. The motors turned in response to zaps of tiny electrical pulses from the tip of a scanning tunnelling microscope. Successive zaps propelled the nanocar across a copper surface. Third generation motors contain a tweak that makes it even easier for nanocars to move over surfaces. More recent motor designs turn faster too. In 2014, a nanomotor capable of spinning at more than 12 million revolutions per second was unveiled.
Nanocar stretch and challenge
If you are looking for a challenge for learners, the 2012 Olympiad paper features a question on the world’s smallest powered car – a ‘nanocar.’ (Olympiad 2012, Round 1, Question 5).
Nanocar stretch and challenge
To challenge the most able, the 2012 Olympiad paper features a question on the world’s smallest powered car – a ‘nanocar.’ (Olympiad 2012, Round 1, Question 5: rsc.li/2qgEBWF).
Fuelled by chemistry
As well as motor shape, Ben’s team have experimented with different motor fuels. They have made molecular motors powered by electricity, UV light and visible light. Ben explains the attraction of using light as the energy source for nanomotors: ‘Light is a very clean energy source and you can fine tune exactly how much energy you put in through the wavelengths of the light.’ But in nature most motors are powered by chemical fuels, and Ben is starting to follow biology’s lead by building artificial nanomotors fuelled by chemicals.
This is a concept that Manchester’s David has been pioneering for a while, with a molecular motor design that looks nothing like Ben’s. Instead, David’s motors are comprised of two large, intertwined, molecular rings known as catenanes.
Seeing shapes of molecules
All too often, students see organic molecules drawn in 2D with 90° bond angles. Although these help them to understand the connectivity and structure of a molecule it can leave them without a clear understanding of how the molecule exists in 3D.
Use of molecular model kits from an early age can help students to see structures in 3D. However, to further enhance this understanding there are a number of excellent online resources available.
Using the ‘Explore your own molecules’ option on ChemTube3D students can explore the 3D shape of organic molecules either loaded from a database or drawn and inputted independently.
A similar resource that uses known crystal structures is the WebCSD Free Teaching subset provided by the Cambridge Crystallographic Data Centre. Students can explore the structure of a number of alkenes on the database using the series of supporting worksheets on Geometric isomerism put together by Newcastle University.
Seeing shapes of molecules
All too often, students see organic molecules drawn in 2D with 90° bond angles. Although these help them to understand the connectivity and structure of a molecule it can leave them without a clear understanding of how the molecule exists in 3D.
Use of molecular model kits from an early age can help students to see structures in 3D. However, to further enhance this understanding there are a number of excellent online resources available.
Using the ‘Explore your own molecules’ option on ChemTube3D (chemtube3d.com/ALevel.html) students can explore the 3D shape of organic molecules either loaded from a database or drawn and inputted independently.
A similar resource that uses known crystal structures is the WebCSD Free Teaching subset provided by the Cambridge Crystallographic Data Centre. Students can explore the structure of a number of alkenes on the database using the series of supporting worksheets on Geometric isomerism put together by Newcastle University (bit.ly/2EvjPHM).
In 2016, David published the first example of an autonomous, chemically-fuelled, molecular motor. ‘We’ve shown for the first time how catalysis can lead to directional motion in a synthetic molecular system,’ David says. The molecular motor catalyses the breakdown of a chemical fuel – the chloroformate ester Fmoc-Cl.
‘It’s the synthetic equivalent of the way most motor proteins are powered in biology,’ he explains. Motor proteins convert chemical energy into mechanical energy by catalysing the hydrolysis (breakdown) of adenosine triphosphate (ATP) to adenosine diphosphate (ADP). Bonds broken in the process release energy to power the motor proteins.
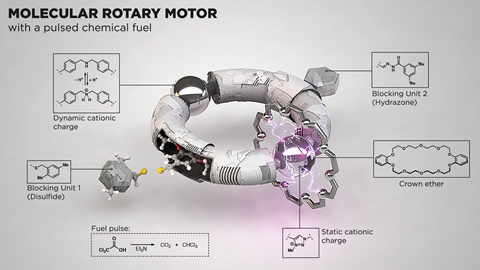
In David’s design, a smaller ring moves along a larger ring that acts as a track. The smaller ring spends most of its time docked at one of two locations on the larger ring. Functional groups on the track act as ‘stop’ or ‘go’ signals controlling the timing and direction of the smaller ring’s movement. ‘The system works through information transfer: the position of the smaller ring on the track determines how fast a temporary blocking group originating from the fuel is attached to the track. It adds more quickly behind the ring than in front, so the forward direction is on average less hindered than the backward direction and so the ring moves forward by biased Brownian motion,’ explains David. ‘One ring rotates about the other as long as unspent fuel remains.’
In October 2017, David’s team introduced another design that works along both circular and linear tracks. The chemical fuel for this set-up is trichloroacetic acid. ‘A cool feature of these motors is that the only byproducts are carbon dioxide and the common solvent chloroform,’ explains David.
His team are currently trying to couple two of their major research interests: molecular motors and synthesis machines. So-called synthesis machines are artificial molecular machines that can synthesise other molecules. They mimic ribosomes – biological machines within cells that build proteins. ‘We are currently trying to incorporate these motor mechanisms into molecular machines we developed previously for synthesis,’ explains David. ‘By doing so we hope to one day be able to power molecular robots to build other molecules and carry out other tasks.’
A new nano- era
‘Synthetic molecular motors are important because they are the power packs for nanotechnology, the equivalent of what batteries and electricity do in our everyday world,’ says David. Learning how to build better nanomotors ‘is a prerequisite for the coming era of molecular nanotechnology’.
Visualising the nanoscale
At around age 11–14, students need to know how to compare ‘nano’ dimensions to typical dimensions of atoms and molecules. However this can be difficult to visualise. The Scale of the Universe resource allows students to start with objects they recognise, eg a human being, and then zoom in to investigate increasingly small objects down to the size of a neutrino (1 × 10-24 m). (This resource requires Adobe Flash).
Further support this learning with the Nanotechnology resources from the Inspirational chemistry collection.
Visualising the nanoscale
At around age 11–14, students need to know how to compare ‘nano’ dimensions to typical dimensions of atoms and molecules. However this can be difficult to visualise. The Scale of the Universe resource (htwins.net/scale2) allows students to start with objects they recognise, eg a human being, and then zoom in to investigate increasingly small objects down to the size of a neutrino (1 × 10-24 m). (This resource requires Adobe Flash).
Further support this learning with the Nanotechnology resources from the Inspirational chemistry collection found in Learn Chemistry (rsc.li/nanotech).
Ben’s team are exploring potential technologies that could use their nanomotors. ‘The number of possible applications is endless,’ says Ben. Stimuli-responsive materials, or smart materials, that change their physical properties when hit with a light or electrical signal is one type of technology Ben’s team are exploring. Soft robotics is another.
‘Soft robotics is an area where I see a lot of opportunity,’ says Ben. Today’s robots are made from hard materials such as metals and plastics. By contrast, the booming field of soft robotics is aiming to make robots from soft and flexible materials that are more analogous with biological systems. ‘One goal is building these motors into polymer networks to make responsive polymers that can contract and expand like muscles,’ he explains.
In December 2017, Ben announced a significant breakthrough. ‘We published on a tiny, artificial muscle powered by our motors that can bend and stretch and lift up a piece of paper just by the action of the motor and the light energy which drives these motors.’ First, a water-soluble version of their motor assembles into nano-sized fibres. These then further assemble into bundles to make centimetre-long strings that are 95% water. The strings flex in response to UV light, lifting a 400 mg piece of paper. Visibly this work is particularly impressive, because you can see the movement with the human eye. ‘When you switch on a lamp and see something you have developed start moving, it’s a delight,’ Ben exclaims.
Article by Nina Notman. Teaching resources by Catherine Smith, a chemistry teacher at Hinckley Academy and John Cleveland Sixth Form Centre.
Downloads
Specification links: molecular motors
Word, Size 55.25 kbSpecification links: molecular motors
PDF, Size 46.07 kbFull article
Word, Size 45.6 kbFull article
PDF, Size 71.29 kbStereoisomerism worksheet
Word, Size 94.33 kbStereoisomerism worksheet
PDF, Size 0.1 mbStereoisomerism worksheet: answers
Word, Size 64.34 kbStereoisomerism worksheet: answers
PDF, Size 0.1 mb















No comments yet