Since its discovery, laughing gas has played its part in our dental surgeries, operating theatres and - more controversially - at our parties
- Nitrous oxide - laughing gas - was discovered in 1772 by Joseph Priestley
- It has been used in anaesthesia since 1844
- Its recreational use has recently seen a revival
Its discovery
Nitrous oxide (N2O), also known as laughing gas, was first discovered in 1772 by Joseph Priestley. A key step towards this was the design of experimental apparatus to collect gas over water, by Stephen Hales in the early 1700s. But Hales' concept of what constituted a gas was quite different from what we know today. He thought that all gases were forms of air. If they wouldn't support life, he believed that the air was loaded with toxic particles. And if they caught fire, the air was contaminated by invisible, flammable particles. That gases are substances in their own right was first recognised by Joseph Black in his classic 1750s investigations into the nature of magnesium oxide, carbonate and their connection with carbon dioxide.
Priestley utilised Hales' apparatus and Black's concept of a gas - though he described them as 'airs' until his dying day - to discover, or report on, a host of gases including: NO, NO2, N2, HCl and N2O (all in 1772), O2 (1774) and SO2 (1775).1 Of course he didn't use these formulae, and indeed his names for them differ markedly from those of today. Nitric oxide (NO) was to him 'nitrous air', and nitrous oxide was 'nitrous air, diminished', reflecting his preparative method of allowing NO to stand in contact with moist iron filings.
2NO + H2O + Fe → N2O + Fe(OH)2
This was however probably not the first appearance of the gas. It may have been prepared, unwittingly, earlier by Black. He published little of his own work, but accounts of his lectures survive and one from 1768 records: 'Ammonium nitrate: is the most fusible of the common salts; when the heat is increased it [is] copiously converted into a vapour; the degree of heat sufficient for its fusion is that of boiling water; if exposed to sudden heat [it] undergoes a deflagration.'2 This is, of course, the way we prepare nitrous oxide in the classroom today.
NH4NO3→ N2O + 2H2O
While Priestley was clearly perplexed as to the nature of his diminished nitrous air, he moved on to other investigations, leaving the study of the gas in the hands of others.
Spotting its medical potential

In 1798, Humphry Davy was appointed laboratory superintendent of the Pneumatic Institute in Bristol, UK. this was an establishment founded on the belief that the recently discovered gases might have curative applications. Here he set to work on his monumental text on the history, chemistry, physiology and recreational use of nitrous oxide - published in 1800 when he was just 21 years old. Curiously, given the purpose of the Pneumatic Institute, the use of this gas in therapy is barely mentioned: a couple of accounts of its use on paralysed patients, and that's about the extent. It is at the end of this book that he makes his oft-repeated statement about the possible use of nitrous oxide in surgery: 'As nitrous oxide.appears capable of destroying physical pain, it may probably be used with advantage during surgical operations in which no great effusion of blood takes place.'3
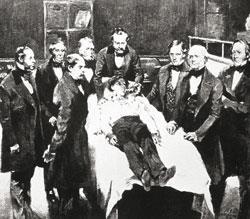
But general anaesthesia by the inhalation of nitrous oxide wasn't demonstrated for over 40 years: in December 1844 by US dentist Horace Wells. But that's not to say that its ability to render the mind unconscious wasn't tried before that. It was.
At a meeting of the Askesian Society (a debating club for scientific thinkers) in March 1800, William Allen, a chemistry lecturer at Guy's Hospital in London, breathed the gas, and described the reaction of the observers. 'The company said that my eyes were fixed, face purple, veins in the head very large, apoplectic stertor [noisy, heavy breathing]. They were all much alarmed, but I suffered no pain, and in a short time came to myself.' His friends thought he was having a stroke, and terminated the experiment. Putting this into the medical context of the time, unconsciousness - especially accompanied by Allen's appearance - boded ill.
Another reason for the delay in realising the anaesthetic potential of nitrous oxide, was simply that that nobody thought to try it. Pain relief was not Davy's main concern, it was barely a peripheral one. The comment in his book was very much a throwaway remark. Davy's interest in nitrous oxide lay in the nature of the chemical bond.
The contemporary belief at the end of the 18th century was that air was a loosely bound compound of nitrogen and oxygen, it supported combustion and - by giving up its oxygen in the body - life. Nitrous oxide also was thought (correctly) to be a compound of nitrogen and oxygen. It supported combustion, and one of Davy's goals was to discover whether it, too, would support life by giving up its oxygen in the body. He found that it did not, hence concluding (again correctly) that there must be some difference between the bonding of the constituents in the two cases.
Slow start for anaesthesia
Even after Wells' 1844 demonstration, uptake of anaesthesia was slow. It was a former colleague of Wells, William Morton, who became its chief proponent and drove anaesthetic use forward. In October 1846, he introduced the use of ether as an anaesthetic.4 Ether had multiple advantages over nitrous oxide, such as being a liquid and therefore easy to transport. It was also, unlike nitrous oxide, already being prepared commercially, in bulk, for other purposes. It is more potent and its vapour is breathed in with air, so avoiding hypoxia (oxygen starvation; another problem associated with nitrous oxide use). It could also take patients to deep planes of anaesthesia for long periods and on waking, they would have no recollection of the operation.
Arguably, though, it was the desire for profit that really caused the boom of anaesthetic use. At the time people were making their fortunes in coal, steel and property, and Morton thought there was money to be made from anaesthesia. To this end he tried to patent both the process, and ether as his anaesthetic agent, something Wells very much objected to. Wells believed that pain relief should be 'as free as air'.
But it wasn't just Morton that wanted to make a fortune. The evidence suggests that the practice of anaesthesia only caught on because other practitioners saw that there was money to be made from charging patients who wanted to use it. As late as the 1880s there was criticism that anaesthetics were not available, for financial reasons, to the pauper inhabitants of work-houses.
Cautious support
The introduction of anaesthetics was not universally welcomed by doctors. In 1847 the great Russian surgeon, Nikolai Pirogoff, described how - being accustomed to the screams and reactions to pain of his patients - he found it repugnant to operate on an inert unresponsive person robbed of his feelings. It was only when he began to realise that the relief of pain removed the surgeon's inhibitions against performing purely palliative operations that he welcomed it.5
Nor were all patients enthusiastic. As early as 28 December 1846, only nine days after the dentist James Robinson had first administered ether vapour for a dental extraction, a patient refused to inhale, having heard, he said, that 'I sent people to sleep and then took out the whole of their teeth'. There is evidence that, at least in the early days, fear of the loss of control that comes with loss of consciousness was not uncommon.
Finding its place
What role does nitrous oxide have in today's modern world? After its re-introduction around 1870 it became the mainstay of dental anaesthesia in the chair until the 1960s, and from the early 1930s it became the principal agent for the relief of pain in childbirth. It has kept its place in anaesthetics - in spite of recent attempts to exclude it - as a 'carrier gas' for more potent general anaesthetic drugs.
The ability to induce feelings of euphoria when inhaled has meant its recreational use has endured for over 200 years (see box). It has also found a new lease of life in its recent introduction - principally in the US - as a fuel booster in the souped-up cars known as 'hot rods', and as an aerosol propellant for whipped cream and cooking sprays.
The club scene
In stark contrast to its use as an anaesthetic, the late 1700s discovery that breathing in nitrous oxide could cause 'merriment' - hence the name 'laughing gas' - was an instant and enduring hit.
It was Humphrey Davy who investigated the effects of breathing nitrous oxide and reported sensations of 'thrilling and pleasure' of varying intensities. He found himself seduced into frequent recreational use of the gas.
Reports show that a few of the people who tried it found it disagreeable, but others had more positive experiences, such as the poet Samuel Taylor Coleridge: 'The first time I inspired the nitrous oxide, I felt a highly pleasurable sensation of warmth over my whole frame... The only motion which I felt inclined to make, was that of laughing at those who were looking at me. My eyes felt distended, and towards the last, my heart beat as if it were leaping up and down. On removing the mouth-piece, the whole sensation went off almost instantly.'6
Laughing gas parties become an immediate success among the British upper classes in the early 1800s. Euphoria and mild hallucinations were the principal attractions, with cost the main drawback.
Several films titled Laughing Gas were made between 1907 and 1920 based on dental use/misuse of nitrous oxide, one featuring Charlie Chaplin. The top-grossing film of 1959 in the UK was Carry On Nurse, in which the cast is somewhat improbably overcome by nitrous oxide in the operating theatre, with hilarious results.
How very retro

The recreational use of nitrous oxide gained popularity again in the 'noughties', when it became fashionable in the club scene. Supplies, ostensibly sold to produce whipped cream, were used to inflate balloons, from which the gas was inhaled.
In New Zealand, a 2002 survey found 12% of first year university students in the country had used it, with 3% inhaling it at least monthly. It was subsequently banned in New Zealand, and suppliers were actively prosecuted. A death in the UK in 2007, presumed due to breathing in neat nitrous oxide - depriving the individual of oxygen - led to calls for more regulation in this country. But it remains popular, with newspaper reports suggesting that Prince Harry has recently tried it.
Risks
There is a risk of asphyxiation if it is not mixed with air, and direct application of cold gas to the lungs can cause significant injury. Additionally, repeated use can cause vitamin B12 deficiency. But if it were located within the framework of the David Nutt - infamously now the ex-chairman of the UK Advisory Council on the Misuse of Drugs - hierarchy of harm, published in 2009, it probably features as one of the less dangerous recreational drugs.7 Its effects, when not those of oxygen deprivation alone, seem mediated by dopamine pathways - the principal reward system of the brain, responsive to all rewards of all kinds, including drugs of abuse.
David Zuck is a retired consultant anaesthetist and a former president of the History of Anaesthesia Society. Pete Ellis is professor of psychological medicine at the school of medicine and health sciences, University of Otago, New Zealand. Alan Dronsfield is emeritus professor of the history of science at the University of Derby, UK
References
- J R Partington, A history of chemistry, volume 3 . London, UK: Macmillan, 1962
- W D A Smith, Br. J. Anaesth., 1972, 44, 297 (DOI: 10.1093/bja/44.3.297)
- H Davy, Researches chemical and philosophical; chiefly concerning nitrous oxide, or dephlogisticated nitrous air, and its respiration. London, UK: J Johnson, 1800 (http://bit.ly/hdnodna)
- B M Duncum, The development of inhalation anaesthesia. London, UK: Royal Society of Medicine Press, 1994
- N Pirogoff, Researches on etherization, St. Petersburg, Russia, 1847
- See ref 3, p515; E M Papper, Romance, poetry, and surgical sleep: literature influences medicine . Westport, US: Greenwood Press, 1995
- http://en.wikipedia.org/wiki/Misuse_of_Drugs_Act_1971









No comments yet