With transport being one of the major sources of anthropogenic CO2 emissions, and transport costs being so sensitive to fuel prices, there is an urgency for car and aircraft manufacturers to reduce the weights of their products, and replace metals wherever possible with plastics and composites.
-
Polymer blends and epoxy adhesives are increasingly being used in the manufacture of cars and aeroplanes
- The Boeing 787 Dreamliner, due to go into service next year, will be made from over 50 per cent plastics
The oil price shock of the 1970s led to the first big increase in the use of plastics in transport. Metal bumpers, wooden dashboards, leather-covered steel steering wheels, and glass headlamp covers were soon replaced by plastics, such as ABS - acrylonitrile (2-propenenitrile) butadiene styrene - a tough plastic made by polymerising 2-propenenitrile and styrene (phenylethene) in a preformed latex of polybutadiene rubber.

Windscreens used to sit loosely in rubber surrounds until chemists discovered that bonding them in place with polyurethane adhesives meant that the strength of glass could contribute to the roll-over strength of a car, so that there was room to reduce the body weight. The rubberised hair in seat cushions was replaced with polyurethane foam (see Box 1), and new applications for plastics and elastomers appeared such as cup holders and sound insulation foam under the bonnet.
Not all were inspired by the desire to reduce the weight of the vehicles - often lower cost or greater design flexibility were more important - but the overall effect was improved fuel efficiency. By 1977 plastics contributed 4.6 per cent of the average weight of a vehicle.1
Car parts and adhesives

Since then the use of polymers in transport increased steadily - in the US toca 7.5 per cent by 2000, and to 8.3 per cent by 2004. In Europe, where fuel prices have traditionally been higher, the figures are up to 10 per cent in some cars. This is significant because every 10 per cent weight reduction yields an improvement in fuel efficiency
of around 5-7 per cent.1, 2
For the near future we can safely predict more rapid increases in the use of polymers, since the parts already replaced vary from manufacturer to manufacturer, and even from model to model. Designers will piggy-back on each other's successes and experiences. There are already vehicles on the road with glass-filled poly(propene) door panels, and others with polyphenene oxide/nylon wings. Noryl, General Electric's blend of polyphenylene oxide and polystyrene, is a strong, hard plastic used in, for example, the wings of the Volkswagen Beetle.
Many cars now have tubular metal frames filled with an epoxy foam that allows greater stiffness with less metal. Indeed, where the need for reduced weight and improved performance outweigh costs, such as racing cars and top-of-the range sports cars - for example, the Porsche Carrera - even the chassis are made of carbon-fibre reinforced composites. Coming soon we will see nylon petrol tanks, and poly-carbonate - a hard, clear plastic made from bisphenol-A and phosgene - replacing glass for sun-roofs and side windows.
The other way that polymers permit a small but significant weight reduction in cars is in joining metal parts together by using high performance adhesives, rather than by riveting or welding. Heat-cured epoxy adhesives can give bonds that are stronger than the steel or aluminium they are joining (see Box 2). Adhesive joints don't have the weak points that are introduced by riveting, and are cheaper and more energy efficient than welding.
Sticky problems
Interestingly, vehicle paints were traditionally based on several layers of different high molecular weight polymers dissolved in solvents. However, because of concerns with solvent emissions, these have now largely been replaced by water-based paints (where the applied polymer is typically of lower molecular weight) or powder coatings (where the polymer is applied electrostatically as a powder to the vehicle, and melted later).
When all the layers have been applied, the vehicle is heated to >150oC to increase the molecular weight of some of the layers or to melt others, and allow them to form a continuous coating. Moves to reduce the energy consumption of this step by lowering the bake temperature would probably lead to problems with the final performance of the adhesives.
However, as more and more of the steel bodywork of the typical car is replaced by plastics or composites the nature of the paint system will have to change. Corrosion protection will become less important, but protection against ultraviolet light degradation of the plastic will be an issue. Paints designed to adhere to metals will generally adhere much less well to polymeric substrates. And, since a 150-180oC bake is not possible for many of the cheaper plastics, some designers are leaving the plastic unpainted, relying on added pigments for colour and ultraviolet protection. If the paint bake cycle is eliminated, the adhesives holding the car together will have to be redesigned, and it may not be possible to maintain the same high-temperature performance under the bonnet.
This leads on to considerations of how plastic panels, particularly the notoriously hard to bond polyalkenes such as poly(propene), will be fixed to one another, or to the remaining metal structures. In recent years several adhesive manufacturers have developed two-part acrylic (esters of propenoic or methyl propenoic acid) adhesives that can bond polyalkenes very well (see Box 3). These are finding application in the car industry but the products are relatively new, so there are no long-term data on the durability of the bonds.
Scrap heap challenge

A factor that is becoming increasingly important for car designers to consider is the recyclability of the materials they use. Most of the steel from scrapped cars is already recycled, but polymers present problems. Simple thermoplastics such as nylon or polycarbonate, once segregated, can be melted down and reused, albeit in less stringent applications, but composites (for instance glass-reinforced poly(propene)) or thermosets present difficulties.
Consider, for example, polyurethane foam seating, one of the biggest uses of synthetic polymers in cars. The cushions from an old car will generally have been compressed or otherwise damaged, so can't be reused. The foams can't be melted down without decomposing, so what can we do?
Three approaches are being used, but none of them is wholly satisfactory.
- Digest the foam in ethene glycol (dihydroxyethane) to yield polyols suitable for synthesising rigid insulating poly-urethane foam.
- Chop the foam up and compress it, and use the resulting material in, for instance, carpet backing.
- Recover some of the energy used to make the foam by burning it in an incinerator.
The first two methods require markets for the products and the third method is not particularly environmentally friendly.
Light aircraft
For aircraft, we find a new set of issues. Material costs are less critical, and recyclability is far less important. On the other hand, reliability of joints is essential (a single failure could be catastrophic). Weight reduction is even more important, as reflected in the plastics and composites contents of the latest aircraft. The Airbus A380 incorporates about 25 per cent by weight of advanced composites, while the Boeing 787 Dreamliner, due to go into service next year, will be more than 50 per cent composite materials.
Adhesive bonding has been used in aircraft manufacture for several decades, since the strong durable bonds that can be achieved with heat-cured epoxies allow the use of light-weight alloys of aluminium and titanium in the construction. However, over the years the demands of the industry on the performance of the adhesives have grown more stringent.
One key area is the toughness of the bond. Even though adhesive joints made with conventional epoxies can be very strong under a steady force, they can fail under impact. Most epoxy adhesives currently used in aircraft are therefore toughened by adding a rubbery material (see Box 4). These rubbery particles have until recently been of the micron scale, but nanoscale tougheners have started to be used.
Using the experience gained in the aircraft industry, car manufacturers are now using toughened epoxy adhesives, in particular to help with crash resistance. One problem for rubber toughened products both in cars and in aeroplanes is that they need to offer impact resistance not just in relatively mild English winters, but also at the much colder temperatures found in the Alps, Eastern Europe and Scandinavia and the American Mid-West, or in aircraft that have just landed from high altitude. Conventional rubbers no longer toughen below about -20o C, so formulators are now working with new materials that keep their rubbery nature down to -40o C.
Fibre-reinforced composites are relatively tough materials, since the fibres slow down crack propagation, but the properties can still be improved. The matrix (often itself an epoxy) can be toughened as discussed. Another approach is to increase the force required to pull individual fibres out of the matrix by improving the adhesion, generally by surface modification of the fibres. Alternatively, highly rigid fibres, such as carbon fibre, can be blended with less rigid fibres that stretch in the presence of a growing crack, and therefore slow down the propagation.
Not all the composites in new aircraft are or will be completely polymeric. A new multi-layered material from Akzo Nobel - GLARE (glass-reinforced fibre metal laminate) - is used on the A380. This material comprises thin layers of aluminium interspersed with layers of fibreglass and bonded together with an epoxy matrix. It is more expensive than either aluminium or traditional composites. However, since the outside layers are aluminium the material can be bonded as a metal, while maintaining most of the benefits of composites, particularly the low density.
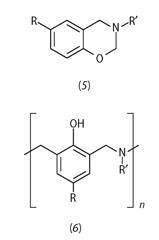
There are also new classes of polymeric binder materials appearing for aerospace composites. The chemicals company Huntsman, among others, is proposing composites based on benzoxazines (see (5) and (6)).4 These are materials that combine the processability of epoxies with the low cost of phenolics, while having certain properties superior to both: chemical resistance, durability in hot/wet conditions, and a low coefficient of thermal expansion (closer to that of metals than that of plastics).
Long-term forecast
Even if fuel costs fall again in the near future, petroleum will eventually become scarce, and replacement liquid fuels (biofuels, oil-from-coal or synthetic fuels using hydrogen from electrolysis) are more expensive without subsidy. The long-term forecast is therefore for relatively high fuel costs. Pressures from green activists, governments and customers for lower carbon emissions from transport are more likely to increase rather than relax.
Weight reduction will therefore continue to be a key objective of manufacturers, and plastics and composites will play an ever increasing role. On the other hand, most plastics are oil-based, so their own costs will also increase. As the polymer content of cars increases, recyclability of the materials will also need to be built in. Whichever way you look, there's an interesting road ahead for polymer chemistry.
David Birkett is a senior scientist with Henkel Adhesive Technologies, Tallaght Business Park, Dublin 24, Ireland.
Further Reading
-
Polyurethanes: D. Birkett, Educ. Chem., 2001, 38 (2), 43.
- Adhesives: D. Birkett, Educ. Chem., 1998, 35 (6), 156.

Box 1 - Polyurethanes
Simple well-known plastics, such as poly(propene) or nylon, are found in modern cars. However, more important contributions are made by polymers with more intricate molecular architectures, such as polyurethanes.
Polyurethanes are formed by the reaction of a di- or polyfunctional isocyanate and a di- or polyfunctional alcohol (polyol):
OCN-R-NCO + HO-R'-OH → -(CO-NH-R-NHCOOR'-O)n-
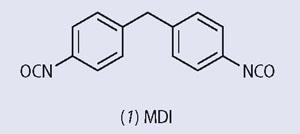
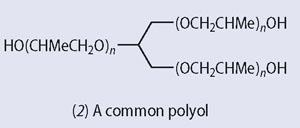
The most common isocyanate used in the preparation of polyurethanes is 4,4´- diphenyl methane diisocyanate (1-iso-cyanato-4-[(4-isocyanatophenyl)methyl] benzene) (1, MDI), and polyols such as (2) and those based on glycerol and propene oxide are well known.
If the 'diol' is water, then the initially formed -NHCOOCONH- group readily loses carbon dioxide to give a urea link,
-NHCONH-. The carbon dioxide forms bubbles in the reacting isocyanate/polyol mix, to give flexible foams used widely in mattresses, packaging, furniture and car seats.
Box 2 - The power of the oxirane ring
Just as urethanes exploit the reactivity of the cumulative double bonds in the isocyanate group, epoxies make use of the strained three-membered oxirane ring. This readily reacts with a primary or secondary amine (see equation (i)).
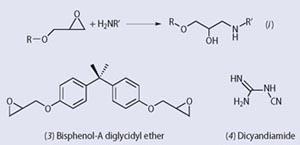
The most common epoxy resin is bisphenol-A diglycidyl ether (3, BADGE). Heat-cured epoxies often use dicyandiamide (DICY, 4) as the amine curative. Below ca 100oC this is a solid which doesn't dissolve in the epoxy resin and therefore barely reacts with it. Formulations containing both are stable at room temperature. However, at temperatures >150oC the DICY dissolves and can cure the resin. DICY-cured epoxies are used as structural adhesives, and form the resin base in many carbon-fibre reinforced aerospace composites.
Most of the joints in a car are now bonded either by adhesives alone or by a combination of adhesives plus a few spot welds or rivets. Likewise most of the hem flanges, where sheet metal parts are bent over on themselves, are sealed with epoxies. For the very low-cost cars that are being developed for emerging markets such as India, it is conceivable that welding and riveting will be eliminated altogether. Curing such adhesives at the elevated temperatures required (>150oC) doesn't pose a problem in car manufacture because such temperatures are routinely used during the paint bake cycle.
Box 3 - Bonding polyalkenes
There is a broad class of adhesives known as acrylics, based on esters of acrylic (propenoic) or methacrylic acid: CH2=CH-CO2R or CH2=CMe-CO2R. These are polymerised by a free radical initiated polyaddition:
X• + nCH2=CH-CO2R → X-(CH2-CHCO2R)n•
Traditionally the free radical, X•, is generated by the reaction of a hydroperoxide, such as cumene hydroperoxide, C6H4CMe2OOH, with a reducing agent such as a substituted hydrazine, a dihydropyridine or a thiourea. The resulting free radical, C6H4CMe2O•, is of the alkoxy type.
Acrylic adhesives bond a wide range of plastics, for instance polycarbonate or ABS, but are ineffective on poly(ethene) or poly(propene) because they can't get a chemical 'grab' on the unreactive surfaces.
However, in the late 1990s a new class of acrylic adhesives began to appear where the free radicals were generated by a different mechanism involving trialkyl boranes, R3B (generally stabilised by complexing with an amine) and traces of atmospheric oxygen.3 This reaction produces alkyl radicals, eg CH3CH2• which are much more reactive than alkoxy radicals and can abstract a hydrogen from a polyalkene surface:
Surface-H + R• → Surface•+ RH
The radical on the surface can now initiate polymerisation of the acrylic monomer, but now the growing chain is chemically bound to the surface.

Box 4 - Fracture toughness
'Fracture toughness', measured in MPam½, is a materials science concept, describing the resistance to crack propagation in a sample containing a crack. Ductile materials such as metals typically have values in the region of 30-50MPam½, while brittle materials such as glass have values below 1MPam½.
Glass illustrates the difference between strength and toughness. Imagine trying to crush a block of glass to powder simply by applying a steady load - the force required would be huge. Now consider what would happen if you tried to tap a nail into it - it would shatter easily, because small cracks once formed can propagate rapidly through it.
Heat-cured epoxy resin is a brittle material. However, if a rubbery material is dissolved in the liquid adhesive, when the material is cured it separates out into small rubbery inclusions. As a crack propagates through such a material the resin between the inclusions can now distort, absorbing energy, and therefore slowing down the propagation. Even holes can function as 'inclusions' - this is part of the reason that natural material such as bone or limpet shells are so tough - they have a porous structure.5
Related Links
Solutions for the composites industry
References
- A. Tullo, Chem. & Eng., News, 2006,84 (24), 12.
- C. O'Driscoll, Chem. & Ind., p18, 26 March, 2007.
- M. Sonnenschein et al, J. Poly. Sci.: Part A: Poly. Chem., 2007, 45, 989.
- Composites World, November 2004
- K. Groshung, New Scientist, 9 June, 43, 2007.






1 Reader's comment