Sonochemistry, the use of sound energy to induce physical or chemical changes within a medium, began with the discovery that simple ultrasonic cleaning baths could be used to influence a range of chemical reactions. Since then the field has expanded outside of chemical synthesis and now finds applications in the food industry medicine, nanoscience and environmental remediation.
-
Ultrasound is used to form and collapse microbubbles in liquid
-
Collapsing bubbles generate high temperature and high pressure conditions that drive physical and chemical changes
Until the mid-1980s the focus of sonochemistry was mainly devoted to improvements in synthesis, in particular reactions involving organometallic compounds.1 Over the past 25 years interest has developed in the subject because of its growing applications in synthesis and the extension of sonochemical studies into different fields such as the manufacture of foodstuffs, cancer chemotherapy, drug delivery, nanotechnology, the extraction of medicinal compounds from plants and environmental remediation.2-5
The power of ultrasound
Ultrasound is classified as sound beyond the frequency that can be detected by the human ear. The normal range of human hearing is between 16Hz and 20kHz. Sometimes called 'silent sound', the ultrasound range is from 20kHz to 10MHz. This range can be roughly subdivided into three main regions: low frequency, high power ultrasound (20-100kHz); intermediate frequency, medium power ultrasound (100kHz-2MHz); and high frequency, low power ultrasound (2-10MHz). The frequency range from 20kHz to ca 2MHz is used in sonochemistry. Frequencies above 3MHz are more commonly used in non-destructive testing and medical imaging.
Sonochemistry is generally performed in a liquid medium. Like any sound wave ultrasound is transmitted via waves which alternately compress and stretch the molecular structure of the medium through which they pass. During each 'stretching' phase (rarefaction), provided that the negative pressure is strong enough to overcome intermolecular binding forces, a fluid medium can be torn apart producing tiny cavities (microbubbles). In succeeding cycles these cavities can grow and then collapse violently with the release of large amounts of energy. Experimental results have shown that temperatures and pressures approaching 5000K and 2000atm are produced during this collapse (Fig 1).
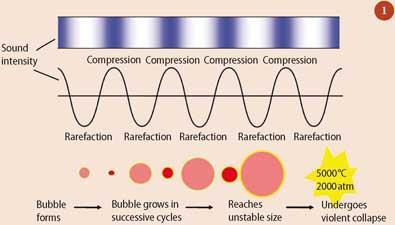
The effects of such 'acoustic' cavitation are best summarised in terms of three different reaction types.
Homogeneous reactions
The mechanical and chemical effects of the collapsing bubble act in two distinct regions: within the bubble itself, which can be thought of as a high pressure and high temperature microreactor; and in the immediate vicinity of the bubble where the violent collapse creates enormous shear forces. The cavitation bubble contains vapour from the solvent or any volatile reagent in the solution. Under the extreme temperature and pressure conditions generated on collapse of the bubble the molecules in these vapours fragment, generating reactive species such as radicals or carbenes. Scheme 1 shows the pathway for the sonochemical decomposition of water into radicals. This process is important in a number of applications. For example, in environmental remediation the hydroxyl radical is a key species in the decomposition of chemical contaminants because it is an extremely strong oxidant and reacts with aromatic compounds. These radicals are also used to kill bacterial cells in sonochemical sterilisation. The generation of radicals can also be used to initiate emulsion polymerisation in aqueous systems, thus reducing the need for chemical initiators.
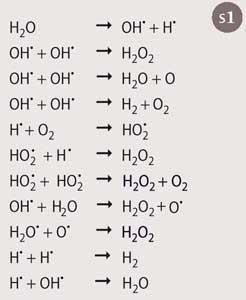
Heterogeneous reactions involving a solid/liquid interface
When the acoustic cavitation bubble collapses close to or on a solid surface it can only do so asymmetrically because the surface provides resistance to liquid flow from that side. This leads to a microjet of liquid being directed towards the surface of the material at speeds of up to 200ms-1 (Fig 2).This effect is equivalent to high-pressure jetting and is the reason why ultrasound is used for cleaning. The jets can be sufficiently powerful to cause pitting of the surface (erosion). This effect can also serve to activate solid catalysts and increase mass and heat transfer to the surface by disrupting the interfacial boundary layers.
Cavitation in powder dispersions causes the solid particles to deaggregate and fragment. This can increase the surface area available for reactions and provide efficient mixing.
Heterogeneous liquid/liquid reactions

In any system involving immiscible liquids cavitation bubble collapse at or near the interface between the two liquids will cause disruption and mixing, resulting in the formation of very fine emulsions. In chemistry such emulsions provide enormous interfacial contact areas between immiscible liquids. For this reason ultrasonic emulsification has been used in conjunction with phase transfer catalysis. This technique has also been used for many years in the food industry for the production of tomato sauce, mayonnaise and other similar blended foodstuffs. Emulsification also has an important role in the plastics industry because a significant proportion of synthetic polymers (over 50 per cent of elastomers) are produced by emulsion polymerisation.
Developments in sonochemistry
Ultrasound is now an established tool in the synthetic laboratory. However, in the past few years developments in sonochemistry have moved beyond synthesis as researchers and industry have begun to find wider applications for ultrasound technologies.
Therapeutic ultrasound
Medical imaging using high frequency ultrasound (ca 5MHz) at low powers is now a routine procedure in diagnostic medicine. At such frequencies and low energies the ultrasonic waves cause no permanent effects on tissue as they pass through the body. In contrast there are several applications of higher power ultrasound in medicine where physical changes do occur. These include physiotherapy using acoustic vibrations to generate heat within the body, dental descaling where the vibrating tip of a dental implement removes plaque from the surface of teeth and the ultrasonic scalpel, which has a blade that vibrates ultrasonically and offers more efficient cutting.6
High power ultrasound can disrupt cell membranes through acoustic cavitation. This is so effective that every biochemistry laboratory is equipped with an ultrasonic cell disruptor which rapidly breaks down cell walls to release the contents. Exposing cells to lower power ultrasound causes a temporary increase in cell membrane permeability, with the cells returning to their normal state afterwards. This is known as sonoporation and can be used to deliver compounds such as drugs or genes into living cells.7
An important feature of ultrasound is that it can penetrate into the human body. Using a system of ultrasound transducers in a concave bowl shape, ultrasound energy can be focused on a malignant site buried deep in tissue. This type of therapeutic ultrasound has two applications. The first is in sonodynamic therapy. In this treatment a patient is given a chemotherapy drug which accumulates in cancerous tissue and is then activated by focused ultrasound. A possible mechanism for this is the sonochemical formation of radicals which initiate chain peroxidation of membrane lipids via peroxyl and/or alkoxyl radicals. The cell membrane is weakened and made more susceptible to the shear forces induced at the focus.8
The second application involves the use of a more powerful array that generates such a concentration of energy that the tumour cells can be killed with sound.9 This is known as high-intensity focused ultrasound (HIFU) and clinical trials are well advanced in the use of HIFU for the treatment of patients with prostate, liver and other soft tissue cancers. In one system that operates at the Oxford Churchill hospital patients lie over a small bath of water in contact with their skin (Fig 3). Two concentric ultrasound transducers in the base of the bath provide the ultrasonic energy. One transmits a low power diagnostic beam, allowing the doctor to visualise the tumour and guide the treatment. The other produces the focused beam.

Nanotechnologies and nanoscience
Sonochemistry is also used to prepare nanosized compounds. The approach was pioneered in the early 1990s by Ken Suslick and his coworkers who sonicated Fe(CO)5 either as a neat liquid or in a decalin solution and obtained 10-20nm size amorphous iron nanoparticles.10 This application is important because the nanoparticles produced are amorphous, not crystalline, and are used as a highly active catalyst for the Fischer-Tropsch hydrogenation of carbon monoxide. A classic method for the preparation of amorphous metal nanoparticles is by the rapid cooling of metal vapour on a cold surface where the cooling rate (106 Ks-1) is so fast that crystallisation cannot occur on solidification. In sonochemistry the rapid decomposition of the metal carbonyl and the very rapid cooling occurs during bubble collapse.
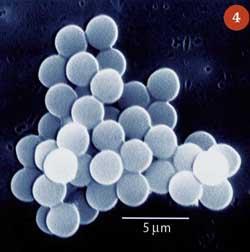
Many different methods using sonochemistry in the synthesis of nanomaterials have now been developed.11 One of the more recent applications is the formation of proteinaceous micro and nanospheres. When an aqueous suspension of a protein, eg bovine serum albumin, is agitated vigorously it forms a foam which slowly collapses. However, sonochemical mixing forms a stable foam.12 The stability is caused by cavitation-induced radicals which cause the protein molecules involved in the foam to crosslink to generate solid, spherical, protein shells (Fig 4). Researchers have developed ways to synthesise such protein shells filled with liquid or gas and these have a wide range of biomedical applications, including their use as contrast agents for sonography, in magnetic resonance imaging and also for the delivery of drugs or oxygen.13
Extraction of natural medicines
In recent years the renewed interest in 'natural remedies' in the Western world and the continued interest of drug companies in the active constituents of natural remedies, as leads for new synthetic drugs, have driven research to find new ways of extracting medicinal compounds from plants. Sonochemistry has been found to increase efficiency and shorten extraction time for a range of plant-derived compounds.14 The 'jet' produced on bubble collapse provides excellent solvent penetration into the plant material and disrupts cell walls to release the contents into the bulk medium.
The compounds in the flowers, leaves and stems of sage (Salvia officinalis) have wide-spectral antimicrobiological activities. Extracts are used to cure and prevent inflammation in the mouth and in upper parts of the respiratory system, skin diseases and sometimes stomach inflammation. Using aqueous ethanol as the solvent, the extraction of active compounds from sage including cineole, thujone and borneol can be increased by more than 20 per cent using sonochemistry compared with normal extraction conditions.13
Ultrasonic extraction could open up opportunities for UK farmers to grow high value crops that have specialist uses. One such crop is rosemary (Rosmarinus officinalis) which contains high levels of natural antioxidants such as carnosic and rosmarinic acid. If these antioxidants could be extracted efficiently, they could be used as additives in food, cosmetics and other pharmaceutical products. Ultrasonic extraction for large-scale agricultural crops aims to make the process economic by reducing both the temperature used and time involved. Using ultrasound to extract antioxidants from rosemary at 35oC provides 100 per cent greater yields after 30 minutes compared to values for thermal extraction.14
Environmental remediation
Power ultrasound has found applications in the remediation and disinfection of water, in sewage treatment and the clean-up of soils.15 In water treatment the effects of acoustic cavitation are used to degrade chemical pollutants. Volatile and hydrophobic pollutants are degraded thermally in the 'hot spot' of the cavitation bubble. Compounds that are less volatile and more hydrophilic are decomposed in the liquid surrounding the collapsing bubble by short-lived hydroxyl radicals produced by the decomposition of water molecules. While ultrasound alone shows promise in such applications its combination with advanced oxidation processes, eg ozonation or ultraviolet light irradiation, shows the most promise.16
Sonochemistry can be used to disinfect water through a combination of the shear forces produced and the radicals generated by cavitation bubble collapse. The acoustic energy required to kill microbiological material in water is very high but if the temperature is raised the energy required is reduced. A potential application for this technology is in pasteurisation where ultrasound can be used to reduce the temperature needed for the process (thermosonication). This has the advantage that the unwanted flavour change that accompanies high-temperature pasteurisation is reduced.17
Low power sonication can deagglomerate bacterial clusters or flocs making the individual species more available for disinfection. Sonication also weakens cell membranes making them more porous (sonoporation) and sonochemistry is therefore very effective when used in conjunction with a biocide.
As towns and cities grow so does the need for more effective sewage and wastewater treatment. The classic method for sewage treatment involves anaerobic digestion, which is effectively a fermentation process in the absence of oxygen. Bacteria in the sludge digest the organic content, reducing the bulk and generating biogas. Sonication can enhance this process by partially degrading the sludge through cavitation, which breaks down the biomass to the organic feedstock digested by the bacteria. However, care must be taken to avoid over-sonication since this might destroy the bacteria.18
An emerging problem affecting land usage is the removal of contamination from sites before they can be developed for building or recreational use. Conventional methods for removing chemical contamination, such as polychlorinated biphenyls (PCBs) from 'brown field' sites or pesticides from agricultural residues, is by washing with either water or organic solvents. This approach has proved successful in terms of cleaning the soil but there remains the problem of treating the remaining polluted solvent. Sonication improves the washing process through the agitation caused by acoustic cavitation, which mechanically cleans the surface of soil particles. There is also an additional benefit in that any contaminants extracted into the wash water are then sonochemically decomposed in the same way as any other chemical contaminant. This technology has been developed into pilot and large-scale processing.19
A quiet revolution
Sonochemistry has come a long way from its origins in chemical synthesis, helped over recent years by the increased availability of inexpensive and reliable ultrasonic apparatus. Today the subject has a multidisciplinary approach with chemists, physicists, biologists and medics working together to identify new opportunities beyond pure chemistry. With proven new applications in medicine, nanotechnology, and environmental remediation, industry and academia are turning to ultrasound technology to develop more effective and efficient processes. Sonochemistry is set to provide a quiet revolution in modern science.
Timothy J. Mason is professor of chemistry and director of the sonochemistry centre in the faculty of health and life sciences, Coventry University, Coventry CV1 5FB.
Box 1 - Demonstrating the physical effects of ultrasound
The effects of acoustic cavitation are dramatic and easy to illustrate using a simple ultrasonic cleaning bath. Note: to generate more even cavitation throughout the tank add a few drops of detergent to the water in the bath to lower the surface tension.
- Cavitation effects on a surface. The jet caused by cavitation collapse near a solid surface is powerful enough to puncture holes in a thin material. Dip a 15cm2 piece of kitchen foil into the bath (do not immerse your fingers). After 30s remove the foil and you should find that it is perforated. (This is an ideal test to determine whether your bath is powerful enough to use for sonochemistry.)
- The fragmentation of powders. One of the common uses of sonochemistry is to break up and disperse powders. Place a few pieces of blackboard chalk in water (150cm3) in a 250cm3 conical flask. Dip the flask into the ultrasonic bath and you should see clouds of chalk produced from the surface of the solid. Very quickly the liquid will become cloudy, illustrating how ultrasound can erode surfaces and break down particles to a much smaller size.
- Emulsification. Conventional methods of making emulsions involve mechanical shaking or high speed stirring. Using ultrasound produces very finely dispersed emulsions. Carefully pour water (100cm3) and then methylcyclohexane (50cm3), commercially sold as a thinner for typing fluid, into a 250cm3 conical flask so that two layers form. Dip the base of the flask into the ultrasonic bath, the clearly defined phase boundary between the two immiscible liquids will become agitated. Shortly afterwards a cloudiness will appear in the interfacial region and within one minute an emulsion of the two liquids will form. (It may be necessary to adjust the depth of immersion to obtain the optimum effect.)
-
Ultrasonic degassing. The cavitational effects which are the basis of sonochemical action are also the reason for the extremely effective use of ultrasound to degas liquids. Any dissolved gases or gas bubbles in the medium act as nuclei for the formation of cavitation bubbles. Such bubbles are not easily collapsed in the compression cycle of the wave because they contain gas and instead they will continue to grow on further rarefaction cycles. The bubbles will coalesce in the acoustic field, become larger, and eventually float to the surface. To demonstrate this effect pour some sparkling water into a conical flask and dip the base of the flask into the ultrasonic bath. Immediate degassing will occur, which dies down when the flask is removed. This process can be repeated several times.

References
- T. J. Mason and J. Lindley, Chem. Soc. Rev., 1987, 16, 275.
- M. Ashokkumar and T. J. Mason in Kirk-Othmer encyclopedia of chemical technology, 5th edn. New York: Wiley, 2007.
- T. J. Mason, Sonochemistry, Oxford university primer series no 70. Oxford: OUP, 1999.
- T. J. Mason and D. Peters, Practical sonochemistry, power ultrasound uses and applications (2nd edn). Chichester: Ellis Horwood, 2002.
- T. J. Mason and J. P. Lorimer, Applied sonochemistry. Weinheim: Wiley-VCH, 2002
- L. A. Crum, J. Phys. Conf. Ser., 2004, 1, 13.
- W. G. Pitt, G. A. Husseini and B. J. Staples, Expert Opin. Drug Deliv., 2004, 1, 37.
- I. Rosenthal, J. Z. Sostaric and P. Riesz, Ultrason. Sonochem., 2004, 11, 349.
- T. Yu, Z. Wang and T. J. Mason, Ultrason. Sonochem., 2004, 11, 95.
- K. S. Suslick, S. B. Choe, A. A. Cichowlas and M. W. Grinstaff, Nature (London), 1991, 353, 414.
- A. Gedanken, Ultrason. Sonochem., 2004, 11, 47.
- K. S. Suslick et al, Ultrason. Sonochem., 1994, 1, 65
- A. Gedanken, Chem. Euro. J., 2008, 14, 3840.
- M. Vinatoru, M. Toma and T. J. Mason in Advances in sonochemistry, T. J. Mason (ed), Vol 5, pp 209-248. Amsterdam: Elsevier, 1999.
- S. Albu, E. Joyce, L. Paniwnyk, J. P. Lorimer and T. J. Mason, Ultrason. Sonochem., 2004, 11, 261.
- Ultrasound in environmental protection, Advances in sonochemistry, Vol 6, T. J. Mason and A. Tiehm (eds). Amsterdam: Elsevier, 2001.
- T. J. Mason and C. Petrier in Advanced oxidation processes for water and wastewater treatment, S. Parsons (ed), chap 8, pp 185-208. London: IWA, 2004.
- T. J. Mason, E. Riera, A. Vercet and P. Lopez-Buesa, in Emerging technologies for food processing, Da-Wen Sun (ed), chap 13, pp 323-352. Amsterdam: Elsevier, 2005.
- S. K. Khanal, D. Grewell, S. Sung and J. van Leeuwen, Critical Rev. Environ. Sci. Technol., 2007, 37, 1064.
- T. J. Mason, A. Collings and A. Sumel, Ultrason. Sonochem., 2004, 11, 205.






No comments yet