Meet the researchers who have an ingenious idea for making phones thinner
Since their invention in the noughties, smartphone design has been dominated by two trends: thinner and bigger.
The two might sound at odds with one another, but smartphone giants like Apple and Samsung have managed to produce phones with slightly bigger screens in slightly thinner cases again and again. Now, chemists are inching closer to making smartphones even thinner with a trick that could change the game entirely: making the battery and the screen one and the same thing.
Researchers from Hong Kong and mainland China have made a battery that doubles up as part of a full colour display. ‘The battery and the screen are the two components occupying the largest volume in many electronic devices,’ explains Chunyi Zhi, a materials scientist from City University of Hong Kong. ‘Therefore, an ultimate strategy to minimise the volume of an electronic device is to integrate the battery together with the screen.’

In your class
Download the text of this article (MS Word or pdf), all the teaching ideas as a single file (MS Word or pdf).
Batteries are a notoriously tricky part of the chemistry curriculum. Now part of more 14–16 chemistry courses, they are also studied at 16–18 within electrochemistry. Students can make links from this topic to many different areas of their course – understanding batteries needs knowledge of ions and reactivity, as well as areas from physics like current and potential difference.
This article discusses a practical application of batteries. You could use it in class to discuss surface area and life cycle assessments, as well as the chemistry of batteries. It’s perfect to show students an example of chemistry in action and how the material covered in school relates to the ‘real world’.
Rethinking components
The colour displays used in smartphones today are liquid crystal display (LCD) screens. They are made up of little dots of coloured light called pixels. When put together, pixels make up the picture on the screen. The screen needs three things to work: a backlight to light it up; a control system to manage how much light is getting through each pixel; and a filter that gives the pixel its colour. Colour is controlled by mixing light of three different colours: red, green and blue.

The researchers decided to try and make a battery that also served as a colour filter. But how do you make a battery flat like a colour filter and produce red, green and blue light with it?
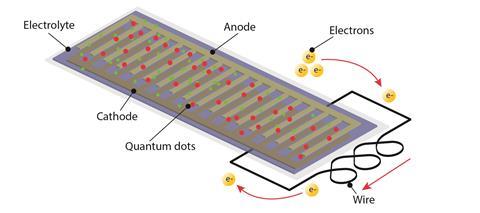
The trick is in the very make-up of the battery. Batteries are chemical cells; they take chemical energy and turn it into electricity. They consist of a positive and negative electrode, called the cathode and the anode, which are connected by a wire and surrounded by an electrolyte. A series of chemical reactions at the electrodes makes negatively-charged electrons build up at the anode. The electrons, which repel each other, escape to the positive cathode. But because they’re separated from it by the electrolyte, they travel through the wire instead. Just like that, an electrical current has been created.
Since the battery is going in a screen, it needs to be flat. For this, the researchers used an ‘interlocking combs’ structure; imagine a cathode comb and an anode comb, stuck together with their teeth fitting in between each other. The researchers then made a gel electrolyte from gelatine, which hindered the electrons generated at the anode from escaping through it to the cathode, but also held the flat battery shape. The interlocking-combs structure means that the battery can fit in perfectly as part of the smartphone screen. ‘Our technology may dramatically reduce the volume of electronic devices,’ explains Chunyi.
A touch of quantum glow
Having managed to make the battery flat, they needed to move on to the second crucial goal: making it give off red, green and blue light. This isn’t something batteries normally do. First, they made sure that the electrolyte was transparent so it would let colours shine through. Then, they introduced tiny particles called quantum dots.
Quantum dots are quite marvellous things. They glow under certain lights – mainly ultraviolet – but the really neat thing is the same quantum dot will glow a different colour depending on its size. This means the researchers could simply choose one type of quantum dot material (cadmium tellurium in this case) and make two different sizes. The smaller quantum dot glows green, and the bigger quantum dot glows red. Easy!
The final stroke of brilliance was that they didn’t need to make blue quantum dots: the gelatine electrolyte gel itself glowed blue under UV light. They managed to make a fully functional, flat battery that could double up as a colour filter by only adding one extra component: cadmium tellurium quantum dots.
There is still some way to go before the tech giants can start to use this screen–battery, however. For example, the battery can’t yet hold the amount of energy needed for a modern phone. Chunyi himself is hopeful though not unaware of the challenges: ‘I do think quantum dot displays are the next-generation display technology, [when we have solved] the problems of stability, price and environment-poisoning,’ he says. For now, at least, the first steps have been taken.

The science behind your phone
Worksheet, ages 14–16
Download this worksheet (word or pdf) that students must complete using their own knowledge and information in this article. It’ll get students thinking about the science behind good phone design and applying their knowledge from other areas of their science lessons. You can also download a teacher guide with answers (word or pdf).
Article by science writer Ida Emilie Steinmark. Resources by Adam Boxer, Jewish Community Secondary School.
In your class
Download this article and all these teaching ideas and the worksheet from: rsc.li/2MI7YiH
Batteries are a notoriously tricky part of the chemistry curriculum. Now part of more 14–16 chemistry courses, they are also studied at 16–18 within electrochemistry. Students can make links from this topic to many different areas of their course – understanding batteries needs knowledge of ions and reactivity, as well as areas from physics like current and potential difference. This article discusses a practical application of batteries. You could use it in class to discuss surface area and life cycle assessments, as well as the chemistry of batteries.
Relevant to your syllabus
Download a list of specification points this article supports from the Education in Chemistry website: rsc.li/2MI7YiH
The science behind your phone
Worksheet, ages 14–16
Download this worksheet from the Education in Chemistry website that students must complete using their own knowledge and information in this article: rsc.li/2MI7YiH. It’ll get students thinking about the science behind good phone design and applying their knowledge from other areas of their science lessons. You will also find a teacher guide with answers.
More recommended resources
- In this practical, students construct a simple chemical cell: rsc.li/2Ce6lUQ
- I ask students to compare and contrast the microscale method for making a chemical cell with a more traditional one and evaluate for effectivity and efficiency. The microscale version is also suitable as an A-level required practical: bit.ly/2IRuluY
- This article has lots of information about the first battery, the voltaic pile, as well as ideas for other activities: rsc.li/2PVrINw
- Here’s another really simple method to build a voltaic pile battery at home or in school: bit.ly/2MOmjpk
More recommended resources
- In this practical, students construct a simple chemical cell.
- Ask students to compare and contrast the microscale method for making a chemical cell with a more traditional one and evaluate for effectivity and efficiency. The microscale version is also suitable as an A-level required practical.
- This article has lots of information about the first battery, the voltaic pile, as well as ideas for other activities.
- Here’s another really simple method to build a voltaic pile battery at home or in school.
Downloads
Article
Word, Size 0.19 mbArticle
PDF, Size 0.19 mbWorksheet
Word, Size 0.27 mbWorksheet
PDF, Size 0.24 mbWorksheet answers
PDF, Size 0.11 mbAll teaching ideas
Word, Size 0.14 mbAll teaching ideas
PDF, Size 0.12 mbWorksheet answers
Word, Size 0.15 mbSpecification links
Word, Size 56.66 kbSpecification links
PDF, Size 49.17 kb










1 Reader's comment