In his seminal work, On the origin of the species, Charles Darwin wrote: Preservation of favourable individual differences and variations, and the destruction of those which are injurious, I have called natural selection or survival of the fittest. In the year that we celebrate the bicentenary of Darwin's birth, it is salutary to consider how much chemistry, in particular natural product chemistry, contributes to what he termed 'survival of the fittest'
-
For lower organisms and plants, the survival of one species over another comes down to chemical warfare
- The interactions between species are often mediated by natural products

At the most fundamental level the success of an organism can be assessed by whether it passes its genes (DNA sequences) on to the next generation. Any genetic changes - and the resultant novel proteins and enzymes - that increase the likelihood of successful reproduction are positive evolutionary events, while genetic alterations that reduce the likelihood of successful reproduction probably move the organism one step closer to extinction.
The ultimate physical consequences of such changes (mutations) may include better means of defence or escape from predators, greater success in mating, or better response to climate change or food restrictions. For most of the lower organisms, eg bacteria and fungi, however, it is the production of natural products that enhances their chances of survival and reproductive success.
Adapt or die
Bacteria have been evolving continuously for around 3.5 billion years. For instance, as the reducing atmosphere of the early Earth (a mixture of ammonia, CO2, methane and H2S) changed to the oxidising atmosphere of the later Earth, bacteria evolved to produce siderophores to help them assimilate iron from the relatively insoluble Fe(III) salts in such an environment.1 Some bacteria have had to adapt to survive hostile habitats, such as the hot sulfurous pools associated with volcanoes, geysers and deep-sea hydrothermal vents. One Thermococcus species, for example, survives in deep-sea hydrothermal vents by producing thermally stable glyceryl ethers as major components of its cell membranes, rather than the less stable but more usual triglycerides (ie esters of fatty acids with glycerol) of terrestrial species.
The myriad fungal species - moulds, mushrooms, yeasts and mildews - that have co-evolved with bacteria over the past 1200 million years often compete with bacteria for food or ecological niche, and chemical warfare is sometimes waged when the species come into contact. The complexities of the chemical compounds involved in these interactions can be bizarre in the extreme, and we can only speculate about the number of discrete genetic changes that have been involved in their 'evolution'. For example, the bacterial species Micromonospora echinospora, found in rock samples from Texas, defends itself from predators by producing the natural product calicheamicin (1). If this substance is absorbed by competing bacterial or fungal cells, metabolising enzymes within these cells react with the trisulfide portion, converting it into a thiol.
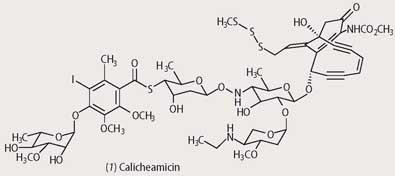
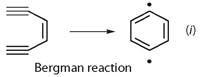
This reacts with the unsaturated ketone part of calicheamicin, which causes a relaxation in the rigid structure, and the ene-diyne part of the structure undergoes a Bergman reaction to produce a benzene diradical (equation (i)). This highly reactive species abstracts hydrogens from the DNA or proteins of the competing bacteria or fungi, resulting in more radicals and resultant chemical damage. (Calicheamicin is currently being investigated as an anticancer agent. The prodrug, Mylotarg, is produced by linking this natural product to a monoclonal antibody specific for the CD33 surface protein of human myeloid leukaemia cells.)
Although there are several similar ene-diyne natural products, this mode of chemical warfare is relatively rare in Nature, and most bacteria and fungi have evolved to produce antibacterial agents which either interfere with the production of new bacterial cell wall material, or they inhibit the production of bacterial proteins and enzymes. The mould Penicillium notatum, for example, produces penicillins, which inhibit the transpeptidation process by which a two dimensional bacterial cell wall precursor is cross-linked to produce the three dimensional final cell wall (Box 1). The mould Cephalosporium acremonium produces cephalosporins which act in a similar way. The natural penicillins, and their relatives the cephalosporins, were exploited by the pharmaceutical industry to provide thousands of man-made semi-synthetic penicillins.
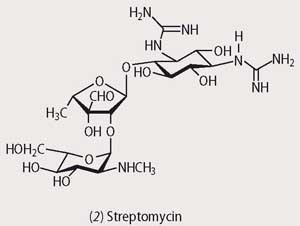
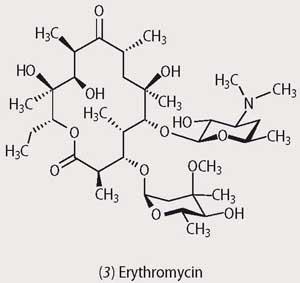
Of all the moulds it is those of the Streptomyces family which have evolved the most ingenious methods for avoiding predation by bacteria, and they produce an arsenal of antibiotics. Among these, the tetracyclines and the aminoglycosides (eg streptomycin, 2) disrupt protein synthesis in the attacking organism by binding to the 30S subunit of ribosomal RNA, while the macrolide antibiotics (eg erythromycin, 3) bind to the 50S subunit and interfere with protein biosynthesis. Throughout the 1950s and 1960s a huge array of natural and synthetic antibiotics based upon the natural products of the Streptomyces family was produced, and these man-made antibiotics together with the penicillins and cephalosporins were thought to be the end for pathogenic bacteria. Unfortunately this was not to be.
Survival of the fittest
A typical bacterium reproduces itself every 20 minutes or so, with perhaps one DNA base pair error in every 10 million (107) for each replication. In a typical bacterial population (in a patient) of 100 billion (1011), the number of possible mutations quickly becomes vast. Any one of these might confer a slight advantage for the bacterium (or may lead to its demise), and given the pressure that the pathogenic bacteria came under once the man-made antibiotics were introduced only, as Darwin predicted, would the fittest survive.
Within a few years of their introduction, bacteria developed methods of resistance to each new antibiotic. Some produced enzymes that could destroy or modify the man-made drugs; others developed methods for expulsion of the drugs - eg efflux pumps; while other bacteria developed alternative ways to make cell wall material that circumvented the routes disrupted by the drugs.
The bacteria were given every chance to mutate and improve themselves since they not only met these new drugs in humans, but also in countless millions of farm animals which had been treated with antibiotics to improve their health during intensive growth programmes. Probably one quarter and perhaps as much as one half of all antibiotic use in the last half of the 20th century was in agriculture, and this was exacerbated by prescribing these valuable drugs to people with colds and other viral infections - for which they are useless. In this way the pathogenic bacteria encountered man-made antibiotics on a huge scale and had to mutate or die.
Within three years of the introduction of the penicillins in 1943, the first resistant strains of bacteria had appeared, and most of these employed enzymes - β-lactamases - to destroy the essential four-membered lactam ring of the penicillins (see Box 2). It is likely that these enzymes had always existed in certain bacteria as part of the long-standing warfare between bacteria and moulds, but the enzymes now appeared across a wider range of pathogenic bacteria. Very quickly many of the simpler penicillins became useless.
At this point another mould metabolite came to the rescue, ie clavulanic acid (4) from Streptomyces clavuligerus. This natural product had evolved over the millennia to combat the effects of the natural β-lactamases since it works as a suicide substrate for this enzyme. In the 1960s Beechams used this natural defence mechanism to counter the effect of the β-lactamases through a strategy that used a combination of clavulanic acid and ampicillin (5), which was called Augmentin. The clavulanic acid serves as a suicide substrate for the β-lactamases, thus allowing the ampicillin free access to the bacterial cross-linking enzymes (transpeptidases) to inhibit cell wall production. While this is a good example of human ingenuity versus bacterial mutation, it is probably only a matter of time before the pathogenic bacteria mutate to find a way of inactivating clavulanic acid.
The pathogenic bacteria found an even simpler route for modifying aminoglycosides such as streptomycin and gentamycin. Over a period of about 10 years from their introduction, many bacteria underwent genetic changes that led to the production of acetyl transferase or phosphoryl transferase enzymes which acetylated amino groups and phosphorylated hydroxyl groups of the antibiotics as they were administered. The resultant acetylated and phosphorylated drugs were either inactive or much less active than the parent compounds.
What makes the bacteria so effective at overcoming man-made antibiotics is not only the brevity of the replication timescale and their rapid rate of mutation, but also their ability to pass on their resistant genes to other bacteria that they encounter in the mammalian gut and other places. This is why it is so important to finish a course of antibiotics - any bacteria left alive at the end of treatment could survive (in the gut) to pass on their resistant genes to bacteria that do not yet have them. In this way gut bacteria can quickly become multiply resistant to many antibiotics. This is the problem that faces us at the present - most of the really dangerous bacteria have acquired resistance to many if not all of the current armoury of antibiotics. It has taken barely 60 years for these lowly organisms to overcome the chemical ingenuity of mankind with their own ingenious chemistry.
Plants and insects
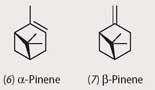
Plants and insects have also been continuously co-evolving for the best part of 300 million years, and once again their interactions are often mediated by natural products. The tree family Pinus is among the oldest in evolutionary terms, and the various species are well protected against insect predation through the production of monoterpenes like α- and β-pinenes, (6) and (7), myrcene, and 3-carene which act as feeding deterrents for most insect species. However, numerous species of the bark beetle family Dentroctonus have evolved during a long period of interaction with pines to produce oxidative enzymes that detoxify the monoterpenes to produce, for example, verbenols (8) and verbenone (9) from α-pinene. These and other metabolites act as aggregation pheromones for several species of Dentroctonus, thus enhancing their ability to breed and survive.
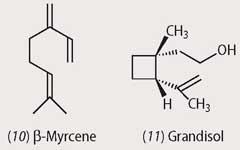
A similar successful subvention is seen in the interactions of the cotton boll weevil with its host plant. The cotton plant produces β-myrcene (10) as a feeding deterrent but the boll weevil uses this compound as the starting material for the biosynthesis of grandisol (11), which acts as an aggregation pheromone. At present we do not know what enzymes have been produced through mutation of existing genes, but there is no doubt that this particular species has evolved to occupy a particular ecological niche through the use of some clever synthetic chemistry.
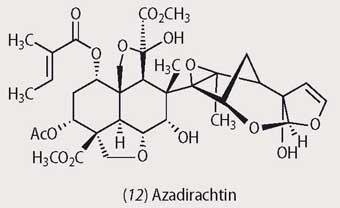
One natural product that is almost universally successful as a feeding deterrent is nicotine, from wild tobacco Nicotiana sylvestris. This plant species can also increase its production of the alkaloid by >200 per cent if it suffers mechanical damage through insect feeding. A few insect species have evolved to produce detoxifying enzymes and others have learnt how to feed without producing major mechanical damage, but nicotine remains a successful chemical deterrent strategy for tobacco plants. The Indian neem tree similarly produces a successful deterrent, azadirachtin (12), which will even deter swarms of locusts. The large number of mutations that must have been required to produce this complex natural product provides strong evidence that survival in the face of insect predation is a major driving force in plant evolution.
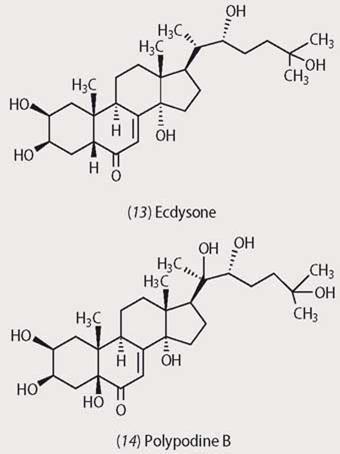
While these are all passive means of defence, plants have also evolved a number of more offensive strategies. Some plants, for example, produce phytoecdysones, ie steroidal compounds which mimic the biological activity of natural insect moulting hormones like ecdysone (13), first isolated from the pupae of the silkworm moth (Bombyx mori) in 1954. Insects feeding on plants producing phytoecdysones like polypodine B (14) suffer disruption of their usual life cycles.
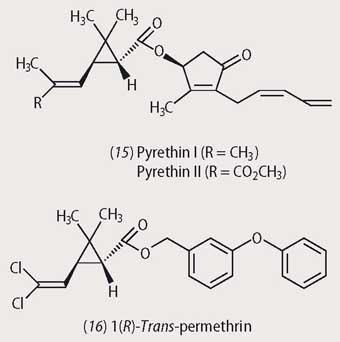
A number of plant species also produce insecticides and the pyrethrins from certain chrysanthemum species are the most potent and broad spectrum. Numerous synthetic analogues of the natural pyrethrins (15), like permethrin (16) have been synthesised, and these are used all over the world as potent and environmentally acceptable insecticides.
And Man?
Darwin made no comment about natural selection in Man and the emergence of Homo sapiens as the dominant hominid owed more to the mental powers of a large well-developed brain than it did to natural product chemistry. However, there are several examples of enhanced survival that can be linked to a particular genetic mutation and the concomitant changes in the chemical properties of a protein or enzyme.
A single change in the DNA sequence of the gene coding for the haemoglobin β-chain (the haemoglobin molecule comprises an aggregate of two α-chains and two β-chains) produces 'sickle-cell haemoglobin' HbS. This aberrant molecule, has a hydrophobic valine in place of a hydrophilic glutamate in the β-chain, and is less soluble in the blood than the normal haemoglobin molecule. The 'sickle-cell haemoglobin' (named after the rigid sickle shape of the blood cells) is less efficient at carrying oxygen around the body, and an individual who inherits copies of this gene from both parents is usually afflicted with life-threatening anaemia. Historically, and even today in developing countries, these unfortunate people usually die before adolescence and so do not pass on their aberrant genes to their descendants.
However, individuals who carry one gene for sickle-cell β-chain and one gene for normal β-chain, can lead reasonably normal lives. Interestingly, sickle-cell haemoglobin cannot sustain the parasite Plasmodium falciparum which causes malaria, so these affected individuals actually have an enhanced chance of surviving in countries where malaria is endemic. In this way the aberrant gene is maintained in the population and currently an estimated one third of the native population of sub-Saharan Africa carry it.
Although natural selection for mutated human genes has occurred in the past, it is unlikely to be a major factor in Man's future development. Control of our destiny is now determined more by economics than genetics, and if we do ultimately destroy our environment, the bacteria will be waiting in the wings ready to inherit the Earth from us.
John Mann is emeritus professor of chemistry in the school of chemistry and chemical engineering at Queen's University Belfast, Stranmillis Road, Belfast BT9 5AG.
Box 1 - Antibacterial agents
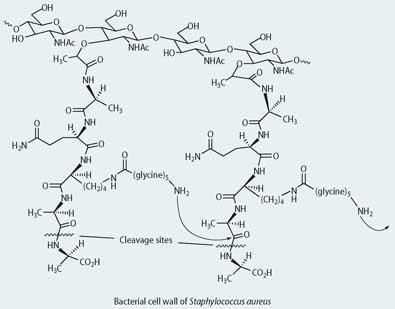
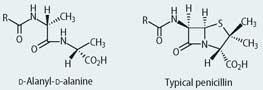
The bacterial cell wall precursor is a polymer comprising a repeating disaccharide unit with attached polypeptide side chains that end with a d-alanyl-d-alanine unit. The transpeptidase enzyme cleaves the terminal d-alanine and the amino group of the glycine then reacts with the penultimate d-alanine on a neighbouring chain to produce the mature cross-linked matrix of the cell wall. The structural similarity between the penicillins and d-alanyl-d-alanine allows the antibiotics to act as inhibitory substrates for the transpeptidase enzyme.
Box 2β - Lactamase attack on penicillin
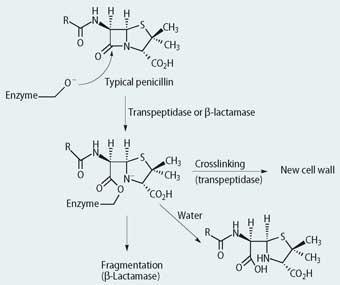
The bacterial transpeptidases and the various β-lactamases are serine proteases and the first stage in their mechanism of action involves attack on the -lactam ring of the penicillin or of clavulanic acid.
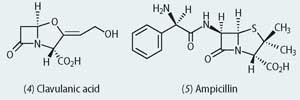
Augmentin is a combination of ampicillin and clavulanic acid.
References
- A-K. Duhme-Klair et al, Educ. Chem., 2009, 46 (1), 25.






No comments yet