In 1958 Rudolf Ludwig Mössbauer, aged 29, published the results of an experiment which gave rise to the branch of spectroscopy which now bears his name. Just over 40 years ago, in 1961, his discovery earned him the Nobel Prize for Physics - one of the youngest scientists to receive this accolade.
The realisation that electromagnetic radiation interacts with matter can be traced back to 1646 when Athanasius Kircher described an 'infusion of the wood lignum nephriticum', which displayed the phenomenon we now call fluorescence. Later in the 19th century chemists discovered other chemicals with this property, including the tricyclic hydrocarbon fluorene (1867), the dye fluorescein (1871), and potassium uranyl sulphate (Henri Becquerel, 1895).
Sodium's green glow
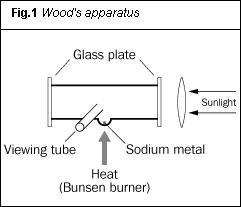
At the turn of the century, physicist Gustav Wiedemann investigated sodium vapour, which he noticed fluoresced in sunlight with a green glow. In an attempt to put this observation on a more quantitative footing American physicist Robert Wood reinvestigated the phenomenon in 1903. Wood used an iron tube sealed at both ends with glass plates. He attached a 'viewing tube' at right angles to the main tube, and a crucible containing the metal sodium to the underside (see Fig 1). Wood heated the sodium to produce sodium vapour, and by focusing sunlight down the tube he observed a 'brilliant green cone of light' at the focus of the lens.1 Using a spectroscope, he discovered the green glow consisted of a 'red band and a green band, the latter appearing distinctly fluted'.
The fact that the fluorescence spectrum bore a close resemblance to the absorption spectrum of sodium vapour was confirmed photographically: '...The two spectra were photographed in contact on the same film, and either one might have been a contact print of the other'. In his 1903 report of his results Wood speculated, 'It will be interesting to see whether the absorption of the vapour is directly affected by the circumstance that it is fluorescing at the same time', but beyond this Wood does not account for the phenomenon he observed.
Wood had, in fact, recorded an atomic/electronic excitation process. Energy was being absorbed by the sodium to form excited atoms. Consequential (but apparently simultaneous) decay enabled the atoms to revert to their ground states. The energies of excitation and decay were identical, as inferred from the similarity of the absorption and fluorescence spectra. This was later termed a resonance effect.
γ-Radiation identified...
Previously, in 1900, Paul Villard had identified γ-rays - ie highly energetic monochromatic radiation - coming from radium. And, following on from Wood's experiment, scientists anticipated that these γ-rays might participate in a resonant fluorescence effect analogous to the interaction between visible light and sodium vapour. We have no record of the number of times this γ-ray fluorescence was sought. Generally, journals publish the results of successful experiments, rather than the absence of results of experiments that don't work. However, in 1929, Swiss-born physical chemist, Werner Kuhn succeeded in publishing the results of just such a failure. He bombarded γ-radiation from 'thorium C' (thallium-208) onto 'radium-G dichloride' (206PbCl2) and, separately, onto 'ordinary' lead dichloride (which contains 52 per cent 208PbCl2). The anticipated nuclear resonance fluorescence failed to occur. The importance of Kuhn's paper, however, lies in his attempt to rationalise his 'non-result'. He noted:
- that the natural, narrow line width of the γ-radiation might be broadened by a temperature effect;
- that there would be an additional broadening, owing to a nuclear recoil when the 208Tl emitted a β-particle as part of the decay process (Fig 2);
- importantly, the effect of nuclear recoil when the γ-ray photon was emitted, commenting...
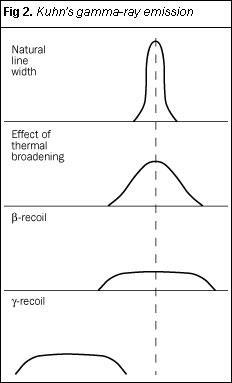
Note that the recoil when the nucleus emits a β-particle is so small as to be imperceptible. This is not the case in γ-ray emission. This arises from the enormous energy associated with a γ-ray photon, compared with that of a single β-particle. Note also that Kuhn did not incorporate a representation of the displacement which occurs when an absorbing nucleus accepts a γ-ray. Mössbauer spectroscopy is the subsequent story of the attempts to overcome the problem of this 'short-fall' in energy.
Success in detecting γ-ray fluorescence came in 1950 by physicist Philip Moon, at the University of Birmingham, UK, who made use of the Doppler effect* to enhance the energy of the incoming γ-ray photons. Moon reasoned that if the γ-ray source could be sufficiently energised by moving it rapidly, then the energy deficit might be made up and resonance would occur. As a source, Moon used a rotor, the tips of which were electroplated with gold. Neutron irradiation in a reactor transformed some of the gold into 198Au, which decays by β- and γ-emission. He then exposed liquid mercury (198Hg) contained in an iron funnel to the radiation from the approaching tip of the rotor. (The funnel contained a Geiger-Müller (GM) detector tube, well-shielded from the 198Au source. Any γ-ray fluorescence occurring in the mercury would be indicated by an increase in the count-rate in the GM tube.) Using an astonishingly high rotor tip speed of 7 x 104cm s-1, Moon recorded 'a small but significant increase in scattering (within the mercury)'.3 He had observed nuclear resonance fluorescence.
At about the same time as Moon was doing his experiments, Karl Malmfors at the University of Stockholm, was trying an alternative approach, which was based on something Kuhn had said earlier. According to Kuhn, the distribution of γ-ray photons might be broadened by thermal effects. Thus by emitting and/or absorbing γ-rays at high enough temperatures, Malmfors reasoned that there might be overlap between the photon distribution from the source and that required by the absorber. He positioned a 198Au source above a pool of mercury, and placed a γ-ray scintillation detector concentric within the pool but well-shielded from the gold source. Malmfors heated the gold with a welding flame, and the detector recorded an increase in count-rate. This led him to report...
The condition for a resonance effect is fulfilled in those cases when the thermal velocity component in the direction towards the scatterer (the mercury) is such that the recoil is compensated'.4
However, though both Moon's and Malmfors' results were positive, they were scarcely outside the range of experimental error. More work would have to be done to convert these beginnings into an acceptable analytical tool.
Box 1. Rudolf Ludwig Mössbauer
The son of a photo-technician, Rudolf Mössbauer was born in 1929. He studied physics at the Technische Hochschule, Munich, where he received his BSc degree in physics in 1953 and his Masters in 1955. After a period of study at the Max Planck Institute, Heidelberg (1955-57), he received his PhD in 1958 and returned as a research fellow to Munich University. He then accepted a fellowship at the California Institute of Technology in 1960. Four years later he again returned to Munich as a professor. By 1972 the focus of his studies had changed and he accepted the directorship of the German/French/British High Flux Reactor at Grenoble, France. Upon subsequent retirement, Mössbauer returned to his beloved Munich, where he now lives.
Enter Rudolf Mössbauer
In 1953 Rudolf Mössbauer, a young postgraduate based in Munich, was assigned a research topic by his supervisor, Professor Maier-Leibnitz. His job would be to take forward Malmfors' recently published work on thermally mediated γ-ray resonance fluorescence. Mössbauer used osmium-191 as his source, which decayed by the emission of β-particles and γ-rays. He passed the γ-rays through an absorber containing either 191Ir or Pt. At the time, the energy of the β-particle was not known and its successful determination would enhance Mössbauer's thesis.
After completing his Masters degree, Mössbauer was persuaded by Maier-Leibnitz to continue with his project as a doctoral student at the Max Planck Institute in Heidelberg. Still pursuing the notion of temperature and the degree of overlap between the absorption and emission lines, Maier-Leibnitz was keen that his student should follow Malmfors' method in achieving overlap at higher temperatures.
Mössbauer, however, felt it would be easier to construct a cryostat and work at the temperature of liquid nitrogen rather than build the high temperature furnace necessary to repeat Malmfors' work. He reasoned that though the overlap would be less, an acceptable temperature dependency might be observed. He anticipated that 'decreasing the temperature should give a reduced overlap of emission and absorption lines, resulting in an increase in transmitted line'.5 (Fewer photons would be scattered, so more would be available to penetrate the absorber and be recorded on the detector.) Mössbauer continued, 'The observation yielded the opposite result'. Increased resonance fluorescence was occurring. Initially he was perplexed by the apparently anomalous result, but he soon realised that he was observing the recoilless emission and absorption of γ-ray photons. He described the phenomenon picturesquely...
This situation (is)...like a person throwing a stone from a boat. The majority of the energy is submitted to the stone, but a small amount goes into the kinetic energy of the recoiling boat. During the summer time, the boat will simply pick up this recoil energy. If, however, the person throws the stone during winter time, with the boat frozen into the lake, then practically all energy is going into the stone thrown and only a negligible amount is submitted to the boat. The entire lake will, thus, take up the recoil and this procedure occurs as recoilless rocess.5
Mössbauer's analogy is illustrated in Fig 3, with the lake frozen. This represents the solid γ-ray emitter (today, usually at room temperature). The recoil energy associated with the γ-ray photon emission is absorbed by the crystal lattice, even at room temperature.
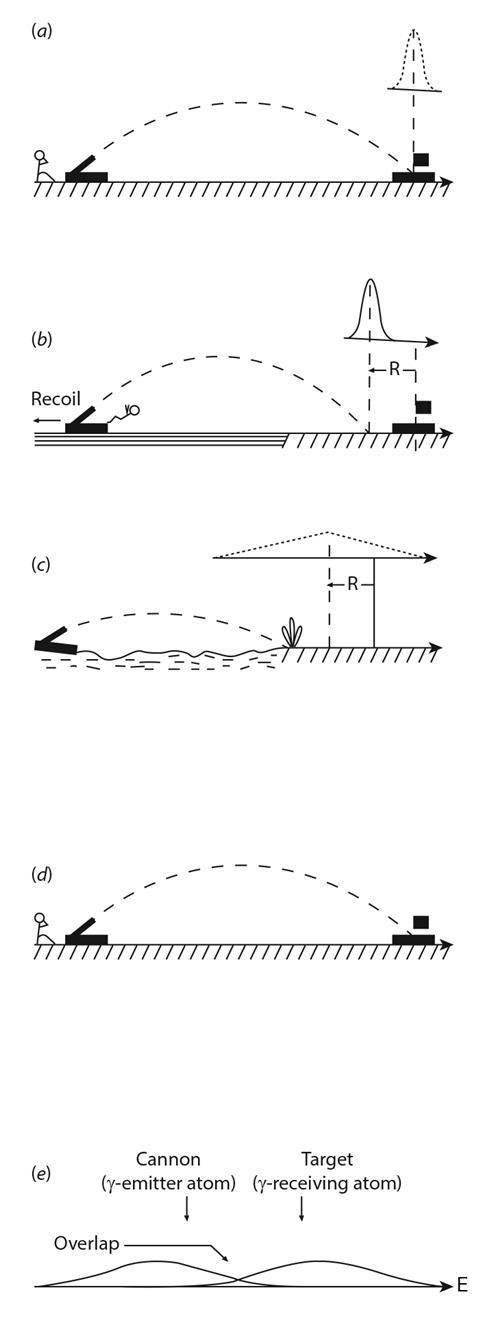
Figure 3. Mössbauer's analogy
Cannon on firm ground firing at target. The bell-shaped curve represents the distribution of the shells around the target.
Cannon is now on the lake. Recoil makes the shots fall short of target by the recoil distance, R.
The cannon is floating on a choppy lake. Recoil occurs and the shells fall short of the same recoi l distance R. Now the motion of the lake causes the distribution of the shells to broaden. This effect is the same as thermal broadening in atoms.
Here the lake is frozen. The cannon cannot recoil, and all the hits are on target, subject to the same distribution as in (a). This represents the γ-emitter at a low temperature. Recoil and thermal broadening are now minimised.
At ambient temperatures, thermal broadening and recoil associated with, both γ-emitting and receiving atoms give minimal resonance fluorescence. Overlap is increased by lowering the temperature of the source and the Mossbauer effect will be observed.
An afterthought?
Mössbauer published his doctoral work early in 1958.6 Scanning through the printed version, however, he realised his discovery could be taken further...
...it occurred to me that I had not performed the main experiment: it should be possible to measure the sharp resonance lines by using the linear Doppler effect. I was so excited, that I dashed across the hallway into the office of Prof. Maier-Leibnitz...and cried 'I take the next train back to Heidelberg, because I forgot about the main experiment.5
Panicking, he realised that he had already sent pre-prints to his main competitors, Professor Moon in Birmingham and Professor Metzger in Philadelphia, US. He thought that with their insight into Doppler shifts applied to resonance fluorescence phenomena, and armed with the conclusions from his paper, he might already be too late. The work he was hurrying to do might even now be 'in press'. But fortune smiled on Mössbauer: neither Moon nor Metzger spotted the potential of Doppler shift extension to his low-temperature resonance work.
The equipment Mössbauer used in his extension work is shown in Fig 4. The source was fixed to a rotating turntable, which served as Moon's gold-tipped rotor, and he chilled the source, turntable and absorber to liquid nitrogen temperature. For expediency, he geared the turntable using toothed wheels from children's construction sets, purchased locally.
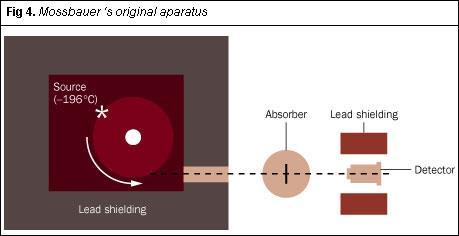
With his hastily-constructed apparatus, Mössbauer recorded the recoilless nuclear resonance absorption of γ-rays by iridium-191 as a function of the linear (tangential) speed of the source. This line (Fig 5) constitutes the first Mössbauer spectrum. His work, incorporating this classic plot, was submitted to the journal Die Naturwissenschaften in August 1958 and was published within a matter of weeks.7 The short paper, scarcely 1000 words long, generated immense interest: within one week Mössbauer received 260 requests for reprints. Its use as an analytical method was soon recognised and this interest sustains: in 2000 Mössbauer spectroscopy featured in 1185 papers.
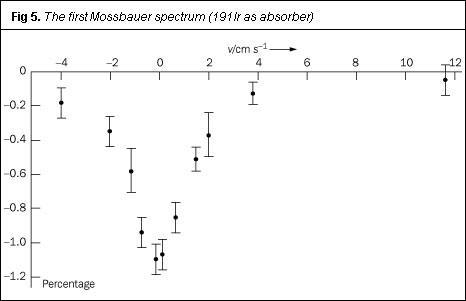
And it all started, essentially, as a bit of a second thought: 'I take the next train back to Heidelberg, because I forgot about the main experiment'....
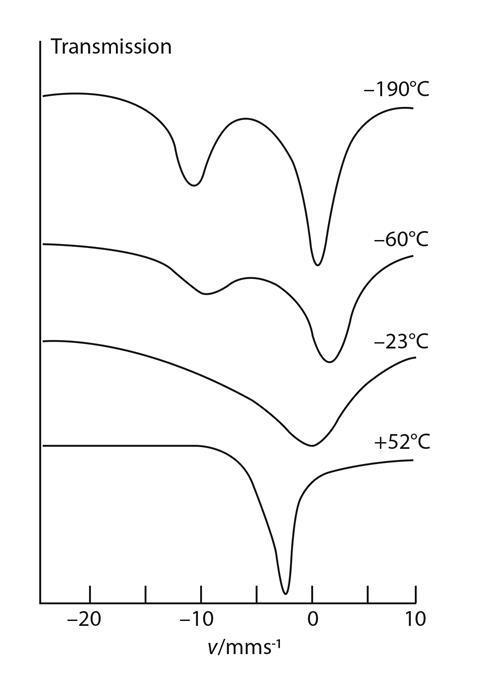
Box 2. Mössbauer spectroscopy in action
Most Mössbauer spectra, even for single substances, are multi-line, owing to effects known as magnetic hyperfine interactions and electric quadrupole interactions.
However, europium-151 gives a single-line spectrum, the position of which depends on its oxidation state. In the case of Eu3S4, at low temperatures, two lines are discernible, one for Eu2+ and the other for Eu3+. One of the earliest applications of Mössbauer spectroscopy was in identifying valency states of metal ions in 'intractable' samples such as slag from copper smelting.9 In the case of europium, scientists observed that at temperatures above 0°C, the two lines merged into a single one with an intermediate velocity shift (see figure). They think that fast electron hopping between the two valency states occurs at these higher temperatures, resulting in an averaged spectrum.l0
In addition to europium, across the Periodic Table there are ca 50 other isotopes that have been used for Mössbauer investigations. Of these, 57Fe and 119Sn feature most frequently. But by far (> 90 per cent) of Mössbauer experiments concern the use of 57Fe. For reasons of quadrupole splitting and hyperflne effects, the signals from iron compounds can be either two- or six-line multiplets rather than singlets. 57Fe Mössbauer parameters (viz, chemical shift, quadrupole splitting, hyperfine field line width and relative intensity of the peaks) form the basis for the applications in chemistry which include the valency state of the iron, ligand attachment, magnetic ordering etc. Depending on the chemical system under study, an investigation can include the dependence of these parameters on temperature, pressure and an applied external magnetic field.
An example of early applications of tile Mössbauer effect involved reconciling identities of 'Prussian blue' made from Fe3+ and [Fe(CN)6]4 and, Turnbull's blue' made from Fe2+ and [Fe(CN)6]3-.The possible reaction products could be (Fe3+)4([Fe(II)(CN)4-6])3 and/or Fe3+(Fe2+([Fe(III)(CN3-6])3 as well as the likelihood of a fast electron transfer which could, in addition, give an averaged electron configuration. However, Mössbauer parameters of the doublets from experiments with the two products as absorbers, confirmed they are Identical, ie (Fe3+)4(Fe(II)(CN)4-6])3.11
Acknowledgement: several texts are available which deal with the theory and applications of Mössbauer spectroscopy,8 and Rudolf Mössbauer has written an engaging personal account of his discovery.5
Dr Jacob Adetunji is a reader in physical sciences in the school of environmental and applied sciences at the University of Derby, Derby DE22 1GB; Alan Dronsfield is professor of the history of science at the same institution.
*The Doppler effect may be noticed when a moving sound source passes an observer. As the source approaches, the frequency is perceived to be higher (apparently more energetic), and when it recedes, it is heard at a lower pitch.
References
- R. W. Wood, Philos. Mag., 6th Series, 1903, 6, 362.
- W. Kuhn, Philos. Mag., 1929, 8, 635.
- P. B. Moon, Proc. Phys. Soc., 1951, 64, 76.
- K. G. Malmfors, Arkiv för Fysik, 1952, 6, 49.
- R. L. Mössbauer, Hyperfine Interactions, 2000, 126, 1.
- R. L Mössbauer, Z. Physik, 1958, 151, 124.
- R. L. Mössbauer, Naturwissenschaften, 1958, 22, 538.
- A. G. Maddock, Mössbauer spectroscopy: principles and applications of the techniques. Chichester: Horwood, 1998.
- J. Adetunji et al, International conference proceedings, Applications of Mössbauer effect, I. Ottalli (ed), 1996, 50, 769.
- O. Berkooz et al, Solid State Commun., 1968, 6, 185.
- W. Kerler et al, Z. Physik, 1963, 173, 321






No comments yet