From building good foundations to memorising the rules, here are some tips to help your students grasp this tricky topic
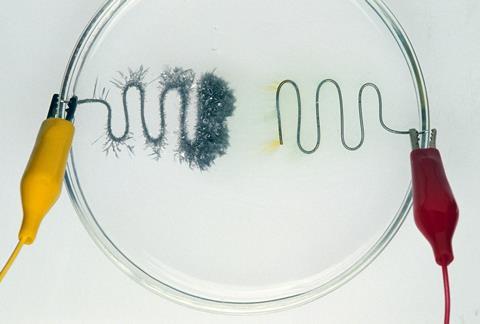
Electrolysis has clear etymology: electro signifying the use of electricity and lysis from the Greek ‘to split, loosen, set free’. The term was coined by Michael Faraday in the 19th century but the process itself was being used to study elements long before that. However those scientists didn’t have to answer exam questions on the topic. And electrolysis is one of the most difficult topics for 14-16 year olds to master.
Why is it so difficult?
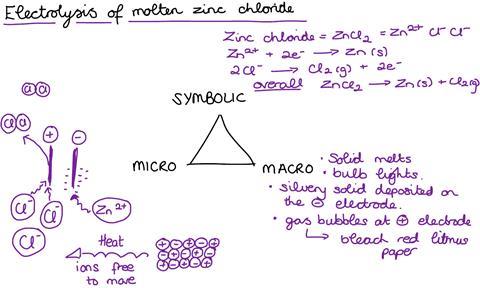
Electrolysis is an abstract concept. By observing electrolysis in the lab, students can see evidence of the products. This might be either through direct observation of a metal depositing or the use of a test, like litmus paper, that can show the identity of a colourless gas. However, what is happening in the molten compounds or solutions is something they need to develop as a picture in their minds. It is a classic example of the power of Johnstone’s triangle in developing understanding.
This mental picture has components in both the microscopic and symbolic domains, each relying on a great deal of prior knowledge. If any part of that prior knowledge is shaky then that can undermine their understanding of the process.
Here are five ideas to help you make electrolysis easier for your students to grasp.
1. Splitting up compounds
Younger students learn that compounds cannot be separated into their elements by physical means (filtration, evaporation etc) and this is a fact they really hold on to. So it can come as quite a challenge to their thinking that electrolysis is able to split compounds into their elements. To overcome this mental barrier, it is first best to acknowledge their correct thinking and then explain that electrolysis does not fit the definition of a physical separation method and so that rule does not apply.
2. Lay the foundations
Before you even try to tackle any new concepts in electrolysis, check your class understands the foundations of the topic. It might seem like going back to these basics is slowing you down in terms of covering the curriculum, but that time will be paid back with more efficient progress later on.
Students need to be confident in their understanding of the nature of ionic charges: just why is that hydrogen ion positive? This helps them with balancing half equations using electrons later in the topic. They should also be secure with the ionic bonding model and how properties of ionic compounds relate to this (specifically with regards to conducting electricity).
Students also need to have a good working knowledge of the reactivity series of metals; which metals are very reactive and which are less reactive. This is important when electrolysis of solutions is introduced, so they can spot whether it is hydrogen or the metal which is discharged when a solution is electrolysed and can also consider if the electrodes are inert.
Students also need a sense of the form different elements take; what they might look like when they are discharged and which are molecular.
3. Slow and steady
It’s a good idea to split the topic into its component parts and teach each bit in depth, recapping prior learning before adding the next layer of complexity. Demonstrations and class practical work can help students form stronger memories, linking the concrete and the abstract. This is especially helpful when it comes to them understanding the movement of ions which can be visualised either by using highly coloured ions – see this Migration of ions practical for how to do this – or with an indicator, as in this Colourful electrolysis activity. Plan lessons carefully to make sure the practical work is purposeful and there is time to link the results to the theory.
It’s a good idea to split the topic into its component parts and teach each bit in depth, recapping prior learning before adding the next layer of complexity. Demonstrations and class practical work can help students form stronger memories, linking the concrete and the abstract. This is especially helpful when it comes to them understanding the movement of ions which can be visualised either by using highly coloured ions – see this Migration of ions resource (rsc.li/2Z2rgUj) for how to do this – or with an indicator, as in this Colourful electrolysis resource (rsc.li/3gsZDtt). Plan lessons carefully to make sure the practical work is purposeful and there is time to link the results to the theory.
Simulations can help students to develop their mental picture in the microscopic domain, at a particle level.
4. Writing half equations
Approaching the writing of half equations boils down to a two-step process:
- Balance the atoms using coefficients as necessary.
- Balance the charges by adding electrons to the correct side.
This is a simple process for an expert like a teacher, but there are lots of places a novice can slip up. Often students start by adding electrons. Many of the gases produced in electrolysis are diatomic, which is easily forgotten by novice students, leading to an incorrect number of electrons in the half equation.
5. Remembering the rules
Some of the rules, like positive ions going to the negative electrode and vice versa, are quite intuitive. Others, especially those about the discharge of anions, are more difficult. There is no substitute for practice here; model your thinking and the questions you ask yourself when you approach an electrolysis question. Sometimes poor recall holds students back so provide knowledge organisers for those whose recall is insecure, then as they become more confident you can remove this scaffolding to check if their ability to remember the rules has improved.
More resources
- Find out about the role of a bioleaching lab technician, Emma, who collects and processes precious metals that are extracted from electronic waste products.
- Try a microscale approach with this practical, complete with integrated instructions.
- Boost your CPD with this teaching guide to electrolysis for 14–16 year-old learners.
- Watch a demonstration and two experiments for learners in this video on the reactivity series of metals.













4 readers' comments