Challenge your students to solve these escape room puzzles by performing simple microscale reactions

Microscale chemistry experiments are quick, easy to perform and can generate discussion about the chemical processes occurring in a drop. Combining microscale chemistry with well-ordered teaching sequences is thought to help reduce cognitive overload.
To help understand chemistry, it has been suggested that students need to work at three levels. These are the macroscopic (observations), sub-microscopic (particles) and the symbolic (equations) levels. This is known as Johnstone’s triangle.
Microscale chemistry is simple to set up and allows more time for students to observe reactions and connect what they are seeing with what is happening to the particles and relate this to symbolic equations.
In this activity, get your students to perform microscale experiments or watch videos of the reactions being performed. Four numbered statements explaining the chemistry of each reaction are provided as downloadable puzzle sheets. The puzzle sheets should be placed in clear plastic wallets or laminated before use.
Students should attempt to deduce which statement is correct. The correct statement number forms part of a four-digit code. This code could be typed into an online form or used to open a locked box to reveal a reward.
Puzzle 1 – Soluble and insoluble
Ask students to perform a microscale precipitation reaction with crystals of two different ionic compounds in a drop of water. A lot of chemistry concepts can be reviewed using this reaction, such as ionic lattice structure, solubility, diffusion and precipitate formation. Lead nitrate and potassium nitrate have been used as an example. This is a microscale version of the golden rain demonstration.

Lead nitrate may be banned from use in some countries, so safer alternatives can be used if required. You should always follow local rules. However, one of the advantages of the microscale approach is that so little of the substance is used that there would not be enough to cause a health issue, and waste can be collected and stored for disposal. Students should wear eye protection and wash their hands with soap and water after the experiment in case of contact.
You will need to give students access to a solubility table to find out which insoluble product is produced in the reaction; in this example it is lead iodide.
PbNO3 (aq) + KI (aq) → PbI (s) + KNO3 (aq)
Puzzle 2 – Thermochromic colour change
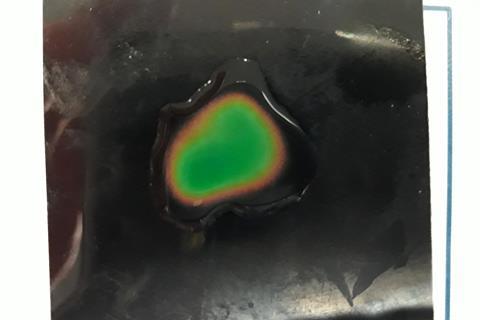
Thermochromic films are composed of sheets printed with thermochromic crystal ‘ink’. The sheets change colour when there is a temperature increase, which can typically be achieved by pressing a hand or finger on the sheet. Ask your students to place a drop of sodium hydroxide solution (2 mol l-1) on top of a thermochromic sheet, followed by a drop of sulfuric acid (1 mol l-1). The reaction is exothermic and will cause a visually pleasing green colour change on the sheet, demonstrating the release of heat into the surroundings.
2NaOH (aq) + H2SO4 (aq) → Na2SO4 (aq) + 2H2O (l) ΔH = -114 kJ mol l-1
Puzzle 3 – Displacement
Get students to place copper wire in a drop of silver(I) nitrate (0.1 mol l-1). A displacement reaction occurs; the products are copper nitrate solution and silver.

The copper atoms lose electrons in an oxidation reaction, forming copper(II) ions:
Cu (s) → Cu2+ (aq) + 2e-
The silver ions gain electrons in a reduction reaction, forming silver atoms:
2Ag+ (aq) + 2e- → 2Ag (s)
The silver atoms are deposited on the copper wire and appear to grow as a forest of attractive looking crystals. A USB microscope, magnifying glass or visualiser can enhance students’ view of this reaction in the classroom.
Puzzle 4 – Conductivity of an ionic substance in solution
Ask your students to insert a simple conductivity meter with an LED into a drop of water. The LED should not light up or will appear very dim. Get them to add a few sodium chloride crystals and the LED will light up as the ions from the salt crystals dissolve and diffuse. This puzzle can be used to discuss various aspects of chemistry such as chemical structure and how the presence of ions in a solution increases conductivity. You can address common misconceptions such as ‘ionic compounds conduct because the electrons can move’ and the presence of ‘sodium chloride molecules’ using this experiment.

Downloads
Escape the classroom - microscale
Handout | PDF, Size 0.12 mbEscape the classroom - microscale
Word, Size 0.45 mb
Escape the classroom

Be inspired to design an escape room experience for students with these chemistry related puzzles
- 1
- 2
 Currently
reading
Currently
reading
Perplexing puddles
- 4
- 5
- 6
- 7






















1 Reader's comment