Peter Borrows takes us on another excursion into local chemistry.
In this issue: calcium compounds - like chalk and cheese
In the last chemistry trail we looked at calcium and its carbonate.1 But we meet many different calcium compounds in everyday life.
Scum and gypsum
Soap scum is a calcium compound. Soaps are usually sodium salts of long-chain fatty acids, such as palmitic (hexadecanoic, C15H31CO2H) or oleic (cis -octadec-9-enoic, CH3(CH2)7CHCH(CH2)7CO2H) acid. The sodium salts are soluble in water but the calcium ones are not. So when soap is used in hard water areas a precipitate of calcium palmitate or oleate forms, ie scum.
Ca2+(aq) + 2C15H31CO2-(aq) → Ca(C15H31CO2)2(s) (i)
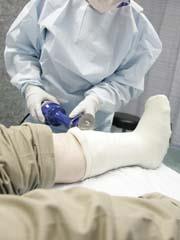
Another widely distributed calcium compound is its sulfate, often mined as gypsum. Unlike the carbonate this is slightly soluble in water and so contributes to the hardness, forming scum as in equation (i). Calcium sulfate is used to make plaster of Paris, CaSO4.½H2O. When mixed with water, this compound expands slightly and becomes hot, forming the dihydrate:
2CaSO4.½H2O(s) + 3H2O(l) → 2CaSO4.2H2O(s) (ii)
You will see calcium sulfate as plaster wallboards and perhaps encasing broken limbs. In an art gallery, you may see sculptures made of alabaster, a crystalline form of gypsum. Perhaps surprisingly, calcium sulfate is sometimes used as blackboard 'chalk' so it is worth checking whether your school chalk reacts with dilute acid.
From bones, through windows, to flares
A walk past the butcher's shop or a dentist's surgery may remind you that bones and teeth are largely hydroxyapatite Ca5(PO4)3(OH). When in a pharmacy or supermarket, if you check the label on toothpastes you may sometimes see they contain calcium carbonate - and even calcium peroxide, CaO2.
Looking through a window, you will be looking through glass, which contains calcium silicate. In the US, calcium silicate is added to table salt as an anti-caking agent.2 However, I have never seen it used in this way in the UK.
Calcium carbide, CaC2, which is much less common today, was used to generate ethyne (acetylene) for fuel lamps, eg on bicycles.
CaC2(s) + 2H2O(l) → Ca(OH)2(s) + C2H2(g)
Mixed with calcium phosphide, Ca3P2, calcium carbide can still be found in self-igniting flares, used at sea,3 because the diphosphane product, P2H4, ignites spontaneously.
Calcium compounds in food
Perhaps you pass a greengrocer's shop. Calcium ethanedioate (oxalate) is found in the leaves of rhubarb (500mg per 100g) with lesser amounts in the stalk. This chemical is also found in spinach (600mg per 100g) and cocoa (500mg per 100g)4 and is the main component in kidney stones and may well be present as crystals in the urine.
Small crystals of calcium lactate crystals may be found in some aged cheeses. In addition, several calcium compounds may be added to cheese and cheese products to enhance flavour, eg calcium phosphate, calcium 2-methylbutanoate and calcium 3-methylbutanoate. Calcium phosphate is an approved food additive, E341, but neither calcium 2-methylbutanoate nor 3-methyl-butanoate appear to be.
References
- P. Borrows, Educ. Chem., 2008, 45 (3), 68.
- K. K. Karukstis and G. R. Van Hecke, Chemistry connections. London: Academic, 2003.
- P. Borrows, Educ. Chem., 2008, 45 (1), 6.
- J. Emsley, Molecules at an exhibition. Oxford: OUP, 1998.






No comments yet