Use these teaching tips to help your students identify and understand electrophiles and nucleophiles in reactions
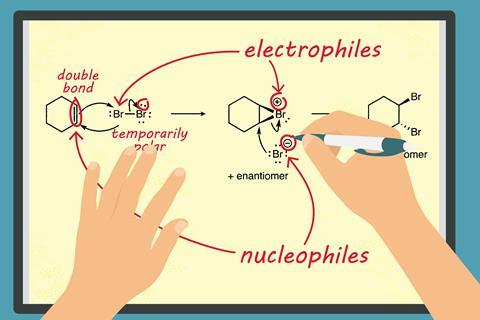
Students need to have a fluency across a range of concepts and skills to gain a secure grasp of organic chemistry. In terms of reaction mechanisms, research has shown that learners frequently have trouble identifying electrophiles and nucleophiles in reactions. It’s particularly difficult for students to recognise the relationship between the pictorial depiction of reaction mechanisms and the underlying understanding of electron-deficient and electron-rich species.
Much research in this area has involved interviews with relatively small sample sizes. In a recent study, researchers evaluated nearly 20,000 written explanations of what occurs, and why, in reaction mechanisms to shed further light on students’ understanding of the processes involved.
The importance of pictorial depictions
Electrophiles, which are electron-deficient, are electron-seeking species. Nucleophiles, which are electron-rich, are nucleus-seeking species. The properties of a given species may be explicit, being depicted pictorially in the form of formal charges, labelled ‘dipoles’ and ‘lone pairs’.
Students perform better in identifying electrophiles and nucleophiles where such explicit features are depicted, but they struggle where properties are implicit (not depicted pictorially). Species with negative charges and/or lone pairs may be easily identified as nucleophiles, while positively-charged species may be identified as electrophiles. Pi-bonds (explicitly depicted) and even some sigma bonds (eg, Al–H bonds in LiAlH4), which can be thought of as shared pairs of electrons, have nucleophilic character and can therefore react with electrophiles.
Teaching tips
- Present students with a complete reaction mechanism and ask them to identify the electrophile and nucleophile.
Then ask them to describe in writing what’s happening step by step and why. Assess this with the researchers’ rubrics. - Show students a mechanism that is missing curly arrows and explicitly labelled reacting species. Ask them to perform the above tasks again.
- Give feedback to develop the sophistication of thier explanations.
- Use these self-assessment activities to improve students’ grasp of reaction mechanisms.
In a chemical reaction, electrophiles and nucleophiles are complementary. An electrophile interacts with a nucleophile during a reaction and vice versa. In previous studies, students were more likely to correctly identify a nucleophile than an electrophile in a reaction. Since chemists need to be able to explain how and why two species interact in a reaction mechanism, it’s vital they can rationalise the roles of both reacting species. This then facilitates the prediction of the products for a specified reaction.
Students are often asked to reproduce a pictorial reaction mechanism in assessments, which can potentially be rote-learned, resulting in poor understanding of the underpinning theory. Instead, the researchers present a strong case for assessing the explanation of the mechanism in terms of the interaction between nucleophile and electrophile.
Being able to explain what’s happening at a molecular level is the key to understanding the processes involved in a reaction mechanism
The authors created a two-part rubric to evaluate the sophistication of students’ explanations about electrophiles. This complements a similar rubric they created for nucleophiles. Students had to describe the sequence of events occurring during a reaction mechanism in terms of the roles of reactants and intermediates, and explain their interaction at the molecular level.
The authors created a two-part rubric to evaluate the sophistication of students’ explanations about electrophiles to complement their nucleophile rubric. Students had to describe the sequence of events occurring during a reaction mechanism in terms of the roles of reactants and intermediates, and explain their interaction at the molecular level.
The researchers categorised the sophistication of student explanations as: absent, descriptive, foundational or complex. Across both electrophiles and nucleophiles, over 54% of responses were classified as descriptive, while nearly 20% were classified as absent, showing room for improvement. Around 54.5% of explanations were at the same level of sophistication for both electrophiles and nucleophiles, and where there was a difference, there was a clear pattern that the electrophile level was lower than the nucleophile level, in line with previous studies.
Being able to explain what’s happening at a molecular level is the key to understanding the processes involved in a reaction mechanism. Providing a narrative to accompany the mechanism will help students correctly draw it from first principles.
Reference
Stephanie J H Frost et al., Chem. Educ. Res. Pract., 2023, 24, 706-722 (DOI: 10.1039/D2RP00327A)
David Read
References
Stephanie J H Frost et al., Chem. Educ. Res. Pract., 2023, 24, 706-722 (DOI: 10.1039/D2RP00327A)













1 Reader's comment