Learn how students predict the location of dipole−dipole forces between two molecules
Intermolecular forces allow us to understand phenomena such as melting points and the folding of biological macromolecules. However, many students struggle to master this chemistry topic – and it’s not just due to linguistic confusion between the sound segments inter- and intra-.
Understanding the origin and location of dipole−dipole interactions, one of several intermolecular forces, relies on a solid understanding of electronegativity. Until now, no study has investigated how students use electronegativity to predict the location of dipole−dipole interactions.
US researchers have investigated how students predict the location of dipole−dipole forces between two molecules. The researchers interviewed 18 first-year university students, providing them with four prompts to respond to.
Teaching tips
- Use the interview prompts from this study to create a good diagnostic test for your students.
- Students often take several incorrect approaches when thinking about dipole−dipole interactions. Become familiar with the approaches detailed in this paper to identify when your students adopt these approaches.
- Many students in the study were comfortable with the concept of electronegativity, but failed to apply it correctly in the context of dipole−dipole interactions, such as in computing differences in electronegativity between unbonded atoms in the same molecule. Be aware of this alternative way students might use electronegativity.
- Present systems with more than one molecule as part of your teaching sequence. This will ensure your students correctly apply the concept of electronegativity, and will further highlight that they should only make electronegativity comparisons between bonded atoms.
- The molecular representation you use when teaching and in questions can affect student thinking. Specifically, the ‘largest atomic size’ approach identified in this paper was only used for molecules that were shown as ball-and-stick or space-filling models, where atomic size is made explicit. Therefore, use multiple representations when teaching to help identify such misconceptions.
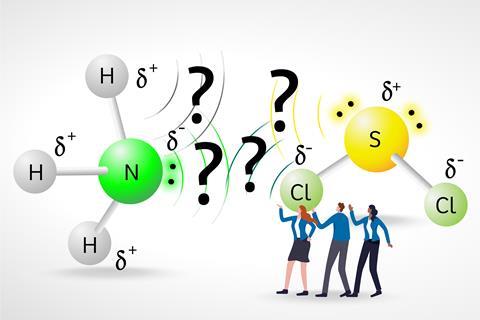
For each prompt, the researchers gave students a pair of simple molecules, such as sulfur dichloride and ammonia, and the students had to show and rationalise the location of the strongest dipole−dipole interaction between the molecules. The molecular representation used for the molecules – chemical formula, Lewis dot structure, ball and stick, space filling – was different for each pair of molecules, although the researchers labelled the atoms throughout.
The researchers analysed the responses and realised that they could categorise students’ rationalisations into one of five general approaches: attraction between opposite charges, electronegativity differences, biggest electronegativity values, largest atomic size and molecular shape.
Dipole reasoning
When approaching the problem by considering the ‘attraction between opposite charges’, students correctly reasoned that dipole−dipole interactions occur between positive and negative ends of a pair of dipoles. They also accurately used differences in electronegativity between atoms to identify polar bonds, and therefore potential dipole−dipole interactions. However, some students who took this approach positioned dipole−dipole interactions within a molecule, and some considered unbonded atoms when comparing electronegativity differences.
There were students who only thought about ‘electronegativity differences’ to rationalise their answer, rather than considering partial charges as the basis for dipole−dipole forces, as in the previous approach. Those who used electronegativity differences, incorrectly identified a polar bond as their answer, rather than a dipole−dipole interaction. Others incorrectly considered pairs of atoms within different molecules.
Some students simply considered the ‘biggest electronegativity values’, without thinking about partial charges. For the pair of molecules SCl2 and NH3, for example, they incorrectly indicated the dipole−dipole interaction between a chlorine and a nitrogen atom, as these have the largest electronegativity values in each molecule.
Another approach students took was to consider only the ‘largest atomic size’. Here, they incorrectly placed the dipole−dipole interaction between the largest atom in each molecule.
Some students focused on ‘molecular shape’, associating it with the incorrect idea that molecules with a bent shape have dipole−dipole interactions between the atoms within the molecule.
Reference
A Farheen et al, J. Chem. Ed., 2024, 101, 3, 766–776 (doi.org/10.1021/acs.jchemed.2c01230)
Fraser Scott
References
A Farheen et al, J. Chem. Ed., 2024, 101, 3, 766–776 (doi.org/10.1021/acs.jchemed.2c01230)













1 Reader's comment