Learning how to use and compare different chemical representations is a vital part of a student’s toolkit
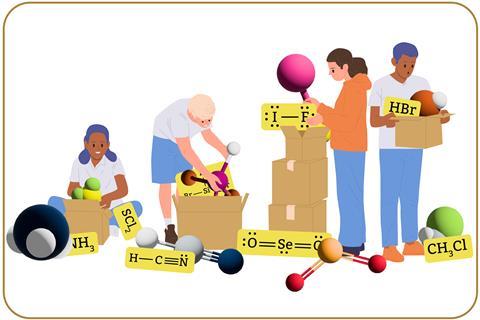
Representations are vital for communicating information about the structure and function of chemical entities. They encode abstract concepts we can’t see by direct observation and present them so we can understand chemical processes and make predictions about chemical behaviour.
Representational competence is the ability to use a range of representations to engage effectively with chemical phenomena. Learners with high representational competence are able to compare different representations and identify information they communicate, implicitly and explicitly. And can also map the features of one representation onto another and explain the relationships between them.
Teaching tips
- Consider the order you introduce representations to learners depending on the topic and what you want them to focus on.
- Question learners about different representations’ features as you use them in your teaching and evaluate each model.
- Shapes of molecules is an ideal topic for comparing different representations. Show the explicit and implicit features of each representation and provide scaffolding, such as graphic organisers, for learners to identify features and generate alternative models from an example.
- Use appropriate representations when teaching intermolecular forces to avoid the perception that they are analogous to covalent bonds.
To make predictions about chemical behaviour, learners must choose or generate the most appropriate representation for what they are trying to communicate. Before applying subject knowledge to a task involving a chemical representation, learners must unpack the representation to consider all the relevant explicit (directly portrayed in the representation) and implicit (hidden) features.
Teaching challenges
How a teacher presents and unpacks a representation influences how a learner unpacks it. Studies show that instructors make assumptions about learners’ understanding of representations’ features. So, it is important to probe learners’ knowledge each time you use representations.
How a teacher presents and unpacks a representation influences how a learner unpacks it. Studies (bit.ly/3YnfrXJ) show that instructors make assumptions about learners’ understanding of representations’ features. So, it is important to probe learners’ knowledge each time you use representations.
Problems can come from unfamiliarity with a particular representation and a lack of awareness of the relationships between different representations. Identifying and using explicit features in one example to generate an alternative representation is challenging for learners. The difficulty increases when dealing with highly symbolic representations, such as chemical formulas, which hold a lot of information you need to fully unpack to draw connections to other representations.
Is order everything?
This study explored the effect of altering the order in which you present representations on the ways that learners unpack their representational features. The study involved interviewing 18 learners selected from a potential pool of 1369 students across seven different chemistry classes.
The researchers presented learners with a set of four pairs of molecules illustrated using different types of representation: chemical formulas, Lewis dot structures, ball-and-stick and space filling models. The researchers gave the learners one of two sets, which had the different representations in opposite orders. Learners viewed the sets of molecules and illustrated their responses to a prompt that stated that the strongest intermolecular force present is a dipole-dipole force. The prompt asked them to draw a dotted line between the atoms in the two molecules that are attracted to each other.
Many learners made effective use of electronegativity values to identify dipoles. Others had the misconception that intermolecular forces are analogous to covalent bonds. Some learners thought that sites bearing lone pairs were unstable and in need of bonding. Some thought a bond would be needed to complete the atom’s octet when lone pairs were not explicitly shown in a representation. The order in which learners received the different representations influenced the features they considered and the representations’ newly explicit features also affected how learners approached the task.
Teaching tips
- Consider the order you introduce representations to learners depending on the topic and what you want them to focus on.
- Question learners about different representations’ features as you use them in your teaching and evaluate each model using this student worksheet for 14–16 year-old learners: rsc.li/4ck40nn
- Shapes of molecules is an ideal topic for comparing different representations. Show the explicit and implicit features of each representation and provide scaffolding, such as graphic organisers, for learners to identify features and generate alternative models from an example.
- Use appropriate representations when teaching intermolecular forces to avoid the perception that they are analogous to covalent bonds.
David Read
Reference
I Nelsen et al, Chem. Ed. Res. Pract., 2024, 24, 815–832 (doi: 10.1039/d4rp00025k)
References
I Nelsen et al, Chem. Ed. Res. Pract., 2024, 24, 815–832 (doi: 10.1039/d4rp00025k)










No comments yet