Microscale chemistry and well-ordered teaching sequences reduce cognitive overload

Practical work is a central feature of science education, and, according to past Ofsted reports, is a key characteristic of the best schools.
Despite this, long debates over the effectiveness of lab work continue. Some research studies have shown practical work isn’t necessarily an effective use of valuable teaching time, and sometimes it does not help students understand concepts.
This is true of my earliest attempt to teach titration to my very first age 15–16 class. I demonstrated the new equipment and required technique first. Then, worksheets distributed, I asked my students to determine the concentration of a solution of sodium hydroxide. Perhaps inevitably, I spent the rest of the lesson going round the room re-demonstrating how to use the new glassware.
As research and good training recommends, I wanted students to make connections between the ‘domain of objects and observables’ and the ‘domain of ideas’ through the activity. But the students didn’t get to practice accurate technique or their mathematical skills much, let alone consider concepts of amount of substance, equilibrium and chemical structure. It was too much.
Chemistry can be overwhelming
To understand chemistry, students need to work at three levels. These are, the macroscopic level (observations), the sub-microscopic level (particles), and the symbolic level (equations). This is known as Johnstone’s Triangle.
As relative experts, teachers have internalised the differences and connections between these levels, and can easily switch between them. Students, as novices, have to contend with all three levels, often at once, without understanding the links between the ideas we are presenting. Simply put, they can find chemistry overwhelming.
Whatever the practical I’m planning, I always work through it from the students’ perspective
Cognitive Load Theory (CLT) offers an explanation for students’ struggles. Originally developed by educational psychologist John Sweller in the 1980s, CLT attempts to explain the cognitive processes related to learning, and develop strategies to increase the likelihood of students learning effectively.
The basis of CLT is that memory is divided into working memory, which has a very limited capacity, and long-term memory, which is effectively limitless. We learn by taking in information from our environment and deriving meaning from it by connecting it with what we already know (ie what is stored in our long term memory).
CLT identifies three broad categories of thinking, or cognitive loads. Intrinsic thinking derives meaning from new information and how it is connected. Dealing with information not related to what you’re learning is called extraneous thinking. Finally, germane thinking is building mental models (or schema) that encode the meaning of the information and how it is connected.
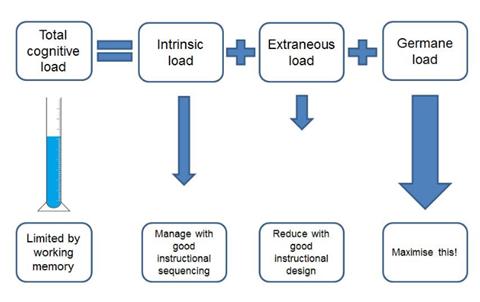
Managing cognitive loads
If the sum of these three cognitive loads exceeds a student’s working memory, then they become cognitively overloaded, and learning stalls. Equally, if intrinsic load isn’t high enough, a student doesn’t develop schema and no learning occurs. So we need to find the balance between providing enough challenge to get students thinking hard, but not so much that they can’t make sense of what we are teaching.
There are various strategies to optimise loads. For example, you can manage intrinsic load through a clear progression of ideas. This helps students make the required connections. You can minimise extraneous load by integrating text and diagrams to minimise the need for ‘instruction searching’. You can optimise germane load by balancing intrinsic and extraneous loads.
There is clearly overlap between these categories, and there’s still a lot of debate about the merits of CLT. But, for my part, it has helped focus my thinking on how I have used practical work in the past, and how I now design sequences of activities.
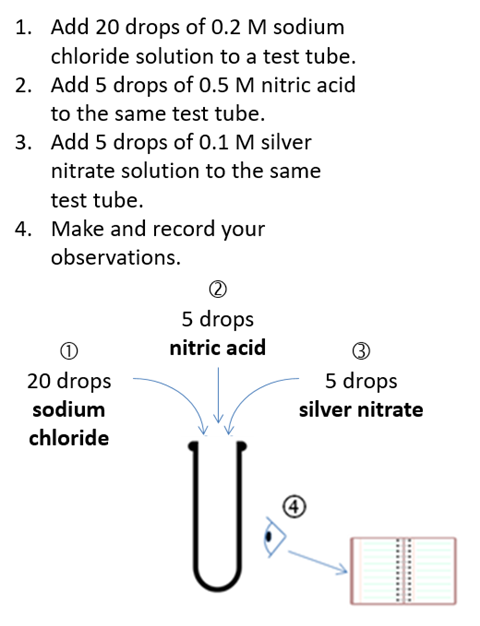
For example, I now use a sequence I developed to teach titration, shown in the box below. It is much more effective than how I was teaching it before. Students successively engage with limited aspects of the wider skill in a conceptually relevant order. The learning environment is more ordered, intrinsic load is improved and extraneous load is decreased. Pupils have more free working memory to focus on building their mental models of titration, maximising germane load.
For example, I now use a sequence I developed to teach titration. It is much more effective than how I was teaching it before. Students successively engage with limited aspects of the wider skill in a conceptually relevant order. The learning environment is more ordered, intrinsic load is improved and extraneous load is decreased. Pupils have more free working memory to focus on building their mental models of titration, maximising germane load.
Microscale chemistry
Thinking about CLT has also led me to increase my use of microscale practicals.
For example, I have often used the standard ‘Nuffield’ apparatus to teach electrolysis. It is fiddly to set up and provides a wide range of potentially extraneous observations and practical issues. Students have to get a powerpack working and connected without shorting. They might spill things while setting up or struggle to collect enough gas for chemical testing.

Microscale activities use much simpler apparatus. Electrolysis in microscale involves two graphite rods sitting in a small drop of copper chloride, connected to a 9 V battery. The observation area is just the drop and tips of the electrodes. This reduces extrinsic load, so students have more working memory to generate meaning from the observations they are making.
You can reuse and extend this practical to teaching displacement reactions with extra drops of potassium halide solution. Adding damp blue litmus paper also links the activity to the acidic and bleaching nature of chlorine. This progression, based on a now familiar experimental setup, helps with the students’ intrinsic and germane load.
Think from a student’s perspective
Whatever the practical I’m planning, I always work through it from the students’ perspective, and pay particular attention to what they’ll be thinking about. This is planning time well spent. I find the cost of missed learning opportunities through ineffective practicals too high not to spend this time in advance.
For each practical activity I plan, I have these questions in mind:
- What is the ultimate mental model I want students to build? (Germane)
- What are the building blocks of this mental model? Have I provided activities to introduce or reinforce these beforehand? (Intrinsic)
- What requirements and observations are likely to distract the student? How can these be minimised? (Extraneous)
After this analysis, sometimes I decide that practical work is not the most effective way of supporting my students’ learning, and I plan something else.
| 1: Recap of neutralisation and use of indicators (KS3 knowledge) | eg ‘Universal indicators’ |
| 2: Carry out a simplified titration | Make the link between neutralisation and accurately measuring amounts of substance, eg ‘The Vinegar Dilemma’ activity |
| 3: Introduce the new equipment | Volumetric pipette and burette – compare and contrast with measuring cylinders and dropper pipettes. Demonstrate good technique, then give the students practice time with deionised water. Highlight good student practice in small groups. Use self and/or peer assessment against a list of requirements (eg vertical burette, clamped safely, use of funnel when filling etc) |
| 4: Introduce the processes of titration | Use the Learn Chemistry Titration screen experiment to allow students to experiment and make mistakes in a low-stakes environment |
| 5: Carry out a ‘standard’ acid-base titration | eg Titrating sodium hydroxide with hydrochloric acid |
| 6: Consolidate data analysis with plenty of examples | eg These example problems |
| 7: Develop understanding of strong and weak acids/bases | Use the resources from the Chemical Misconceptions book |
| 8: Develop investigative skills | Instruct students to plan and carry out an analysis to determine the composition of a solution of unknown composition. |
Further reading and resources
If you’d like to share your experience of applying CLT to practical work, there is an ongoing Twitter discussion at #CogSciSci and you can sign up to a peer-support network at bit.ly/CogSciSciMail
If you’d like to share your experience of applying CLT to practical work, there is an ongoing Twitter discussion at #CogSciSci and you can sign up to a peer-support network here
The New South Wales Centre for Education and Statistics has published a summary of cognitive load theory for teachers: bit.ly/2x9fn1Q
The New South Wales Centre for Education and Statistics has published a summary of cognitive load theory for teachers
The Getting Practical website provides professional development in improving teaching quality through teaching practical science: www.gettingpractical.org.uk
The Getting Practical website provides professional development in improving teaching quality through teaching practical science
The ‘Cognitive Load’ webpage of Notre Dame High School, Norwich, lists a wide range of blogs and research papers: https://ndhsblogspot.wordpress.com/cognitive-load/
The Learning Scientists are a group of cognitive pschycology scientists who produce articles and resources to support teachers and support students learn better: http://www.learningscientists.org
The microscale practical is available as a suggested GCSE practical activity, suitable for all students regardless of the exam specification they are following: www.ocr.org.uk/Images/311750-pag-activity-chemistry-electrolysis-suggestion-2.docx
David is a former Head of Chemistry and Science at schools in North Hertfordshire. After two years working as a Chemistry Subject Adviser at the UK exam board OCR, David returned to teaching Chemistry at Aldenham School, Elstree, and tweets @dave2004b













1 Reader's comment