Help your 14–16 learners tune into the patterns in reactivity and physical characteristics of these groups on the periodic table
Elements in each group of the periodic table share similar chemical properties and show patterns in reactivity and physical characteristics. Understanding these trends helps explain the behaviour of certain elements and their roles in everyday applications.
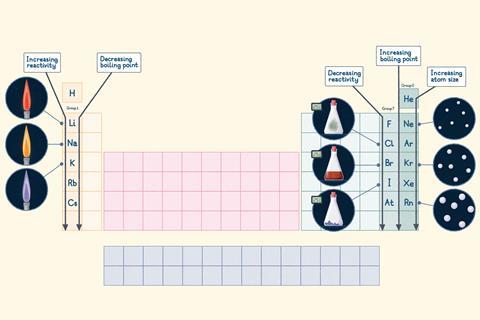
Group 1
Known as the alkali metals because they react with water to produce an alkaline solution (pH above 7).
- Elements: lithium (Li), sodium (Na), potassium (K), rubidium (Rb) and caesium (Cs).
- Physical properties: the group 1 metals are soft and you can cut them with a knife. Lithium, sodium and potassium are less dense than water, so they float. They have low melting points compared to other metals and these decrease as you go down the group.
- Chemical properties: alkali metals are highly reactive; they react vigorously with water. Each metal gives off a characteristic flame colour when you heat it. For example, Li, red; Na, yellow; K, lilac.
- Key trend: alkali metals become more reactive as you move down the group because their atoms get larger. This means the outermost electron is further from the nucleus and the attraction between them is weaker, so the electron is more easily lost.
Did you know …?
Lithium is in demand because it’s used in rechargeable batteries such as those in mobile phones and laptops.
Group 7
Known as the halogens because they react with metals to form salts and in Greek, halogen means salt former.
- Elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I) and astatine (At).
- Physical properties: fluorine and chlorine are gases at room temperature, bromine is a liquid and iodine and astatine are solids. The elements get darker in colour as you go down the group (at room temperature, Cl is greenish-yellow, Br is red-brown and I is dark grey).
- Chemical properties: halogens are reactive non-metals and readily form salts when they react with metals. A more reactive halogen can displace a less reactive one from a compound.
- Key trend: halogen reactivity decreases as you move down the group because their atoms get larger, meaning there’s a weaker attraction between the nucleus and incoming electrons.
Did you know …?
Chlorine kills bacteria, so it’s used to treat drinking water. It’s a poisonous gas and was used as a chemical weapon during the first world war.
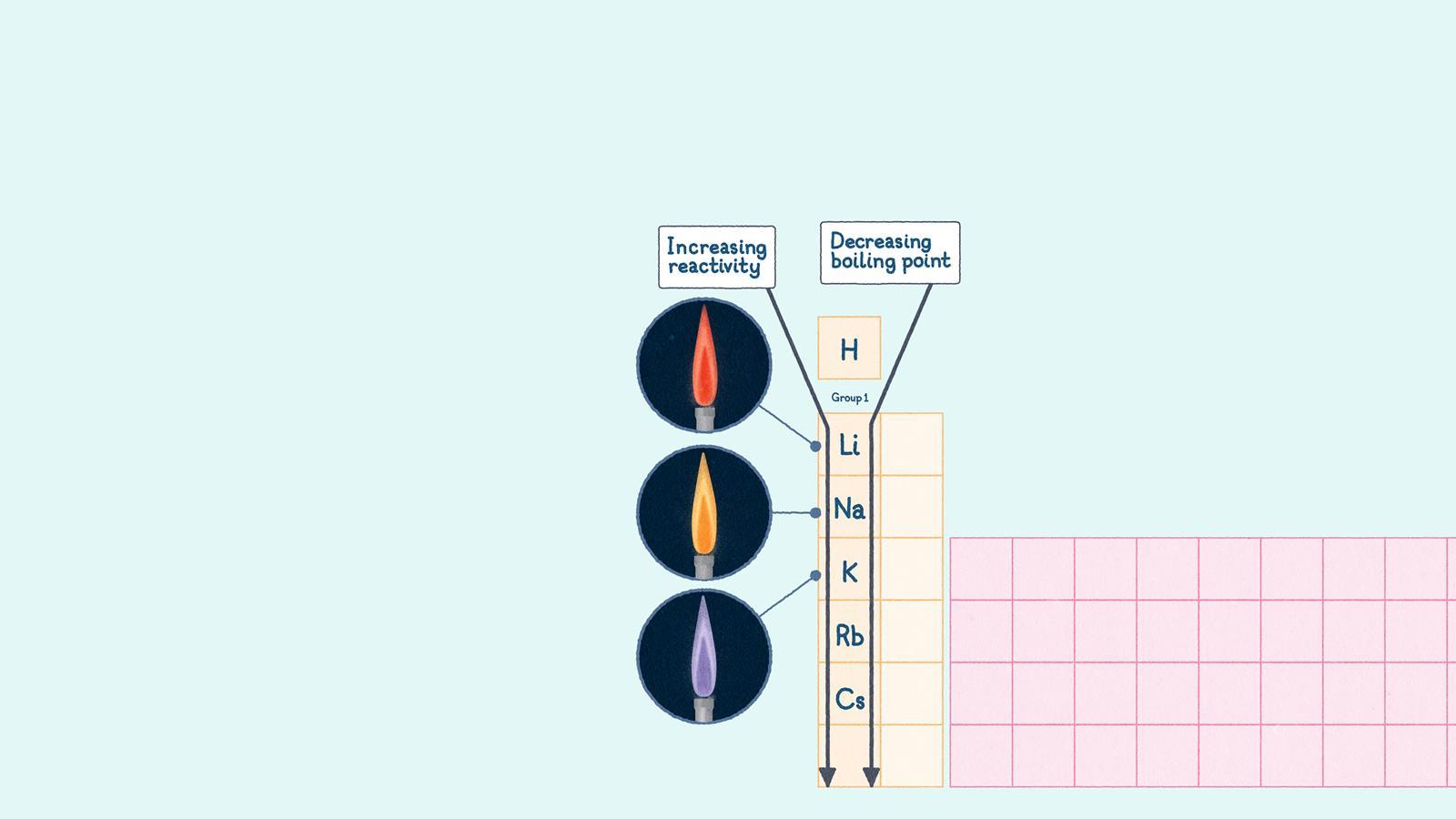

Group 1 chemical properties
Alkali metals become more reactive as you move down the group because their atoms gets bigger. Each metal gives off a characteristic flame colour
Group 7 physical properties
Halogens are different states at room temperature. The elements also get darker in colour down the group
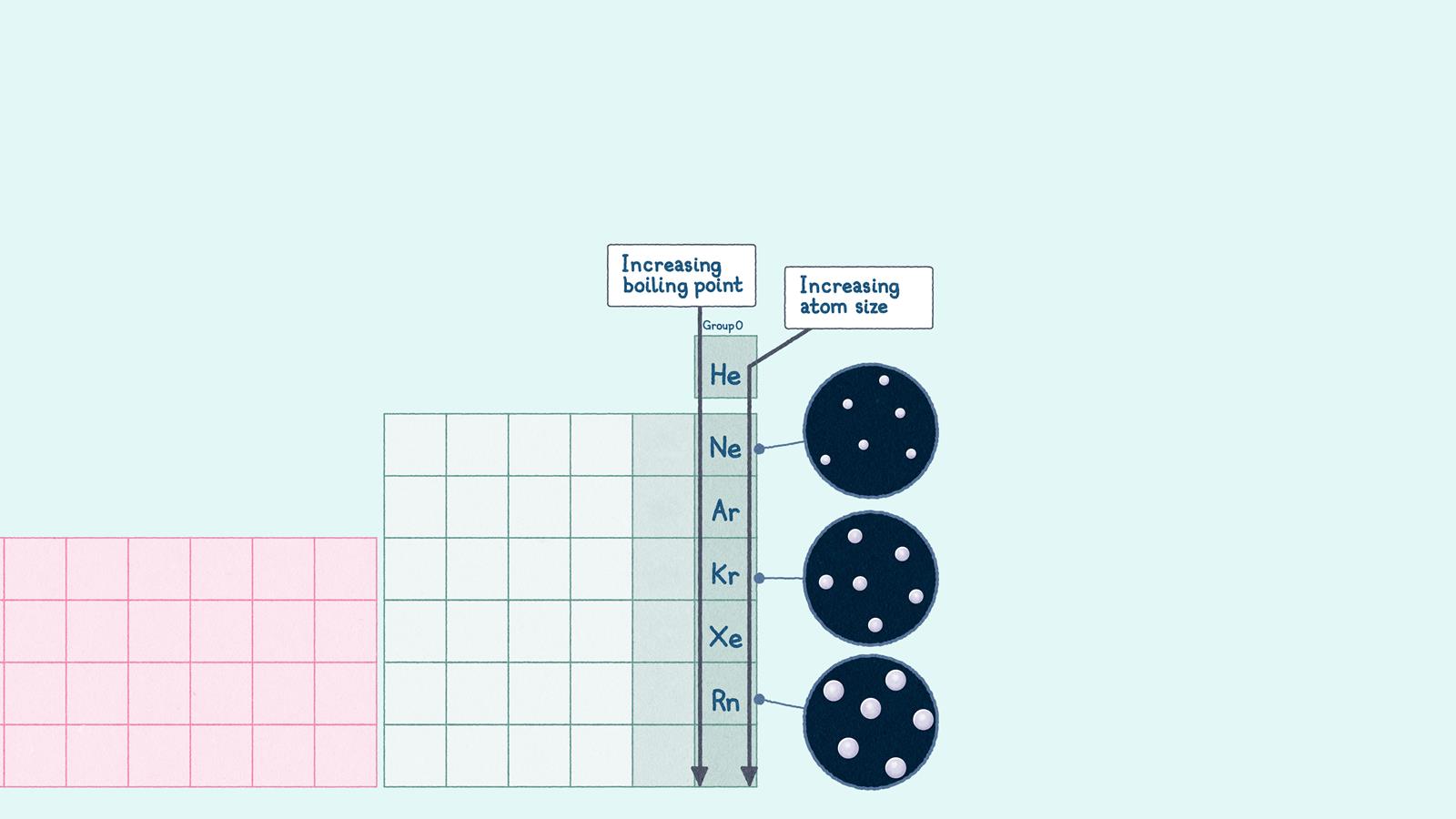
Group 0 physical properties
The boiling points of noble gases increase down the group, because their atoms get bigger
Group 0
Known as the noble gases because they are unreactive and generally exist as unbonded atoms, giving the idea that they’re ‘unwilling to interact’ with others.
- Elements: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe) and radon (Rn).
- Physical properties: all the group 0 elements are gases. They have very low boiling points, which increase slightly down the group.
- Chemical properties: noble gases are very unreactive and unlikely to form compounds under normal conditions.
- Key trend: the boiling points of the noble gases increase down the group because their atoms get larger. Larger atoms have stronger forces of attraction between them and require more energy to overcome.
Did you know …?
Helium is used in balloons and airships because it’s lighter than air, so it floats.
More resources
- Help your 11–14 learners know how to find elements, groups and periods on the periodic table with this poster and activity.
- Banish student confusion about period 3 trends with this handy modelling guide.
- Check out the Royal Society of Chemistry’s interactive periodic table.
- Use these top tips and teaching ideas to teach atomic structure and periodicity at post-16.
Resources written by Louise Glynn
Downloads
Groups 1 7 and 0 trends poster
Handout | PDF, Size 0.85 mbGroups 1 7 and 0 trends fact sheet
Handout | PDF, Size 0.12 mbBecoming Mendeleev student sheet
Handout | PDF, Size 0.77 mbBecoming Mendeleev teacher notes
Handout | PDF, Size 0.7 mbGroups 1 7 and 0 trends fact sheet
Editable handout | Word, Size 0.43 mbBecoming Mendeleev student sheet
Editable handout | Word, Size 0.75 mbBecoming Mendeleev teacher notes
Editable handout | Word, Size 0.69 mb
















No comments yet