Chemists in Spain synthesise a new type of polymer material by stringing together fullerene molecules
It's almost 15 years since Sir Harry Kroto and colleagues published their seminal paper on the discovery of buckminsterfullerene, C60. In that time, these all-carbon molecules with their spherical, or more precisely, truncated icosahedral structure have yet to reveal their killer application. Recently, Nazario Martin and his colleagues at the University of Madrid, have developed a novel electroactive 'fullerene receptor' molecule, a molecule that specifically recognises and binds to the surfaces of fullerenes. Now, the team has taken that research one step further to make hybrid molecules that bring together the fullerene receptors with fullerenes themselves to form linear aggregates of molecules lined up like a string of pearls.1
According to the researchers the recognition of fullerenes by the receptor involves a pincer-like grasp in which aromatic carbon rings on the pincer bind to sections of the fullerene surface.
More technically, the new bucky-ball receptor is a pi-extended analogue of tetra-thiafulvalene (TTF), 2-[9-(1,3-dithiol-2-ylidene)anthracen-10(9H)-ylidene]-1,3-dithiole (or exTTF). The complete receptor is composed of two exTTF units connected through an isophthalic diester spacer. The resulting large and concave aromatic surface of the exTTF units acts as the recognition motifs for the convex surface of the buckyball molecule.
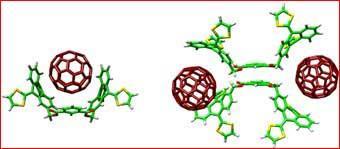
This recognition process leads to a strong bond between the pincer and the pinched fullerene. The team used numerous analytical techniques to demonstrate the chemical structure of the resulting materials, including nuclear magnetic resonance spectroscopy, mass spectrometry, UV-Visible spectroscopy, and atomic force microscopy (AMF). AMF studies revealed that some strings have up to 35 'buckypearls'.
The researchers also point out that under certain conditions, it is possible for electrons to transfer from one 'pearl and pincer' unit to another along the 'necklace', which could endow the new materials with interesting electromagnetic properties for optoelectronic applications.
References
- N. Martin et al, Angew. Chem. Int. Edn, 2008, 47 (6), 1094.






No comments yet