A look at recent chemical science research from Chemistry World
Functionalised fibre catches flu before you do
Scientists in China have developed a fibre that can trap the flu virus, which could be used in face masks and air filters to help to control the spread of the disease.
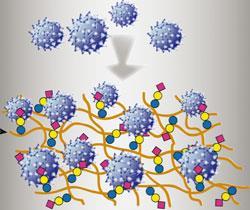
Every year, millions of people find themselves suffering the aches and pains of flu, and in the very worst cases - around 300,000 per year - the infection is fatal. Recent flu pandemics have illustrated the severity and potency of the influenza virus and the continuing threat of future outbreaks demands rapid and effective controls.
When the flu virus infects a host the viral protein hemagglutinin attaches itself to carbohydrate chains on the host's cells. 'This protein-carbohydrate interaction provides an interesting target for antiviral developments,' says team leader Xuebing Li at the Chinese Academy of Sciences, Beijing. His team exploited this well known interaction by attaching the same carbohydrate chains to the natural polysaccharide chitosan, creating a fibre that can trap the flu virus. 'Multivalent presentation of the carbohydrate on a scaffold enhances the virus binding,' says Li, adding 'chitosan's unique biological properties and perfect fabricability make it an ideal scaffold'.
The team then prepared the functionalised chitosan as a filter to measure its ability to remove the avian flu virus H1N1 from water. The filter removed the majority of the virus and was significantly more effective than chitosan alone.
Ben Davis, a chemical biology researcher at the University of Oxford, UK, says this work is a 'neat combination of existing technologies,' and a good example of translational research. 'If we are genuinely going to try to translate discoveries in chemical biology and materials into a functional output,' says Davis, 'then this is exactly the type of [work] we need.'
While initial results are promising, Li admits there is still much to be done. 'We have only verified that the fibre can capture the flu virus in an aqueous medium,' cautions Li. 'Further work is required before a real application such as a face mask or air cleaner filter can be attempted.'
Phillip Robinson
Zap and the aromaticity is gone
German chemists have shown that it's possible to turn off aromaticity with a blast from a laser beam. Tuning the frequency of the laser excites electrons from the ground state to a non-aromatic state of alternating single and double bonds.
Inga Ulusoy and Mathias Nest at the Technical University Munich, performed the work as part of a larger project in Nest's lab. 'This is the first step in a broader project to control the reactivity of molecules in general,' Nest explains. 'The question we ask ourselves is, "Can we use a laser pulse to control the reactivity of a system?" and one of the easiest things for us to try [to control] is aromaticity, because we are theoreticians... so we decided to start with something small.'
'The idea is so simple that one can be angry that one didn't have it oneself,' says Jörn Manz, who works on laser control of reactions at the Free University of Berlin. He describes the work as a new twist in the field of laser control and 'a little ingenious'.
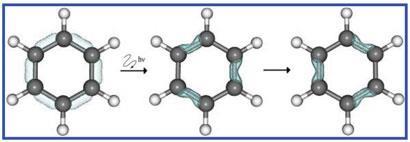
As every student of chemistry learns, benzene is a highly stable compound with six equal bond lengths and delocalised pi electrons in rings above and below the plane of the molecule. However, there are several different
definitions of aromaticity. Ulusoy and Nest decided to work with the definition of benzene as having six-fold electronic symmetry.
The pair identified non-aromatic states of benzene that do not satisfy this definition - one with different electron density on alternate atoms, and one with different electron density on alternate bonds. They then calculated the path and energy required to get to these states.
The non-aromatic states are actually a superposition of the ground state and the first or second singlet state, but a direct transition to either of the two excited states is forbidden by symmetry, so instead an alternative path had to be found via several other excited states. Ulusoy then put a trial laser pulse into her calculations and iteratively optimised the properties of the pulse to excite half of the ground state to one of the intermediate levels, and from the there to the final state.
One of these new states should look very familiar to anyone with a passing knowledge of chemistry. 'The state that we created corresponds to the cyclohexatriene molecule, where we have alternating bond orders of one and two,' says Nest. 'That was our practical definition of a non-aromatic state.' However, the molecule is not quite a true cyclohexatriene - the electron density moves so fast that the atoms can't keep up and the bond lengths are still all equal, much like Kekulé's resonance structures of benzene.
As a first step, Nest admits he is a long way off his final aim of firing laser pulses at compounds so that new reactions can be controlled and performed. However, Manz says he thinks this work should inspire further work in this area.
Laura Howes
References
- X Li et al, Biomacromolecules, 2011, DOI: 10.1021/bm200970x
- I S Ulusoy and M Nest, J. Am. Chem. Soc., 2011, DOI: 10.1021/ja206193t






No comments yet