In many parts of the world biting insects are major disease vectors, being the source of malaria and yellow fever for example, though in the UK they are mainly just a nuisance. Since Roman times various plant oils have been used as repellents but these have largely been replaced by synthetic chemicals, the most successful being DEET (N,N-diethyl-m-toluamide, 1). The subject provides the basis for some interesting synthetic, organic chemistry experiments.

One million people, mainly children under five, die each year in Africa of malaria. Where the health impact is less, the economic cost of biting insects can be considerable.
The largest industry in Scotland, for example, is tourism - the 13-14 million tourists every year (1996) contribute £2000 million to the country's economy. A study by Edinburgh University's Dr Alison Blackwell, however, found that the Scottish midge, Culicoides impunctatus, cost the Scottish tourist industry £286m with 86 per cent of first-time visitors saying they would advise their friends not to travel there in July or August, the height of the midge season.1
Repellents - natural and synthetic
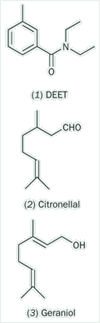
One of the best known natural insect repellents is citronella oil, which is obtained from various Cymbopogon species, eg Cymbopogon nardus. The oil contains high concentrations of citronellal (3,7-dimethyl-oct-6-enal, 2) and geraniol (3,7-dimethyl-octa-2,6-dien-1-ol, 3), both of which are repellents, though the effect lasts less than one hour. Candles containing citronellal are often used outdoors to keep insects at bay but, like unscented candles, are not particularly effective.
Many other essential oils exhibit repellent properties, including garlic, cedar, turmeric, eucalyptus, lavender and clove. A long established repellent ('as used by the Vikings'), derived from the essential oil of bog myrtle (Myrica gale) contains α-pinene, 1,8-cineole, germacrene and α-cadinene.
The drawback of using natural oils as insect repellents is that the composition and quality are variable, and depend on the age of the leaves, the season and the method of extraction. Moreover, many of these substances are potentially phototoxic or allergenic.
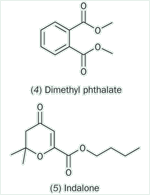
The problem of the variability of natural oils is overcome by using synthetic chemicals. Dimethyl phthalate (4), popular for many years, was first patented in 1929 as a fly repellent and, along with the butyl ester, has been widely used as a general purpose insect repellent. In the 1990s, however, some health concerns came to light when scientists discovered this chemical to be teratogenic (ie capable of producing birth defects in animals and/or humans) and it also proved to be slightly mutagenic in the Ames test. Just before World War II, two new synthetic repellents, indalone (5) and ethyl hexanediol (6) were introduced, followed, in 1953, by DEET (N,N-diethyl-m-toluamide, 1).
DEET is now the most widely used insect repellent and the one by which all others are judged.2 The US Environmental Protection Agency estimates that 200 million people worldwide use DEET each year. However, DEET does have some disadvantages - it is a solvent for plastics (watch faces, pens and cameras), has an unpleasant smell, is somewhat irritating to the skin and mucous membranes, and has been associated with some cases of neurotoxicity, particularly in children. Of recent concern is its potential to enhance the absorption of toxic insecticides and nerve gas agents through the skin.
DEET synthesis
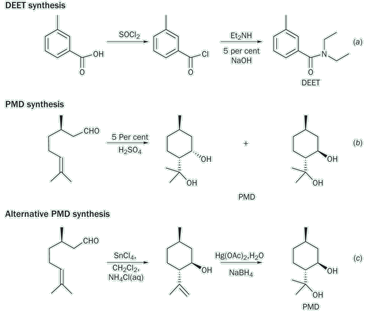
DEET is synthesised (see Box 1) from m-toluic acid by conversion to the acid chloride using thionyl chloride, followed by reaction with diethyl amine under Schotten-Bauman conditions, equation (a).3 The entire process should be done in a fume cupboard because the reagents are corrosive (SOCl2), smelly (pyridine, diethylamine) or lachrymatory (m -toluoyl chloride).
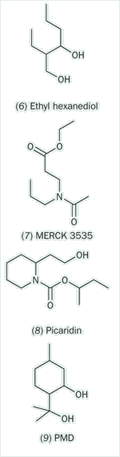
In the 1970s, Merck introduced a new repellent, MERK 3535 (IR 3535 (7)), which has more favourable cosmetic and toxicological properties than DEET. More recently, Bayer developed another amide, Picaridin (Bayrepel, KBR 3023 (8)), which is increasingly replacing DEET. Another potential DEET replacement is p-menthane-3,8-diol, PMD (9), which was originally obtained from the leaves of the lemon eucalyptus tree Eucalyptus (now Corymbia) citriodora but is now produced synthetically. PMD first emerged as an insect repellent in China in the 1970s under the name quwenling or sometimes qwenling, where it has largely replaced the synthetic dimethyl phthalate.
Box 1 - Experimental
DEET
To a 5 ml round bottom flask equipped with a magnetic stirrer bar add m-toluic acid (0.5 g, 3.7 mmol), dry ether (0.2 ml) and pyridine (0.2 ml). Then add thionyl chloride (0.55 ml, 7.6 mmol), loosely stopper and stir for 10 minutes. Remove excess thionyl chloride by evacuation at water pump pressure for 10 minutes. Charge a 50 ml conical flask with diethylamine (1.3 ml, 12 mmol) and 10 per cent aqueous NaOH solution (5 ml) and cool in an ice bath. Add the m-toluoyl chloride using a Pasteur pipette and stir for 5 minutes. Dilute the reaction mixture with diethyl ether (10 ml) and transfer the mixture to a separating funnel. Run off the aqueous phase and extract twice with ether (2 × 10 ml). Dry the combined ether extracts (MgSO4), add toluene (1 ml) and evaporate the solvent to give the product as a pale yellow oil.
PMD
Into a 250 ml round bottom flask place water (100 ml) and add H2SO4 (4 g). When cool add citronellal (20 g) and stir vigorously for 24 hr. Separate the upper layer and extract the aqueous phase with ether (20 ml). Dry the combined organic phases (K2CO3) and evaporate to give the product as a viscous oil which partially solidifies on standing.
PMD synthesis
The synthesis of PMD, equation (b), is based on a recipe first published by Barbier and Leser in 1897,4 and later in the 1950s (see Box 1).5 Pure citronellal is expensive, but can be replaced by Eucalyptus citriodora oil which is available from essential oil suppliers and aromatherapists. The progress of the reaction can be monitored by thin-layer chromatography (silica gel - 4:1 hexane-ether), observing the spots with the p-anisaldehyde - sulfuric acid - ethanol reagent. Citronellal (Rf 0.51) is converted mainly into the PMD isomers (Rf 0.06, 0.10) together with small amounts of isopulegol isomers (Rf 0.29, 0.39). The enthusiastic chemist may want to try an alternative two-step reaction, equation (c), in which citronellal is cyclised to give isopulegol,6 which is then hydrated using hydroxymercuriation - demercuriation to give mainly trans-PMD.7 Students (and teachers) may like to ponder the reason for the different stereo-chemical outcome of these reactions. Since PMD has three chiral centres, there are (23) eight possible isomers, four pairs of diastereoisomers. All of these, some in small amounts, are formed in these reactions and it would be interesting to consider ways of producing the less common ones.
Discovering repellents

There are several strategies for discovering insect repellents. One has been to test a large number of available compounds for their repellent properties. The driving force behind this strategy was the military's need to restrict disease among personnel - often a greater hazard than enemy action. Between 1942 and 1945, the US Department of Agriculture tested over 7000 compounds as insect repellents. By 1956, over 20,000 compounds had been screened, one of which was DEET. The second strategy is to reconsider an old repellent, like bog myrtle, or quwenling, and the third strategy is to design a molecule to interact with the appropriate insect receptors. However, attraction/repellency (see Box 2) do not appear to be simple lock-and-key processes and are not fully understood. Moreover, since insect repellents are now classed as biocides, the EU regulatory authorities require extra toxicology and ecotoxicology tests on animals, in addition to efficacy tests done by entomologists (see Box 3). The latter cost at least £1 million, making the emergence of new products via this strategy unlikely.
Box 2 - Attraction and repellency

There are several different physiological processes at work here. At long range (up to 100 m), receptors in the insect antennae detect volatile chemicals exuded by the human body in the open. Lactic acid and carbon dioxide act synergistically to persuade the insect to fly upwind (ie towards the target). At closer range, other attractive factors operate, not only chemicals (eg 1-octen-3-ol) but also warmth, moisture and dark clothing all draw the insect closer.
Entomologists utilise attractive chemicals, especially carbon dioxide, to attract species for study. For example, ticks live in grassy areas in forests and when hungry, climb to the top of blades of grass to await a passing ankle. When a suitable target brushes past, they attach themselves to the skin and begin feeding. The simple expedient of placing a cloth on the ground with a block of solid carbon dioxide in the centre attracts ticks to the cloth then they can be removed for study. Others use the attractants for darker purposes. The commercial midge eater uses carbon dioxide and 1-octen-3-ol to attract midges which are then sucked into a bag where they dehydrate and die, taking up to a million a day out of the environment.
Repellents appear to act to neutralise the attracting factors, perhaps by blocking the lactic acid receptors, thus rendering the target invisible to the insect. But they also seem to be effective in varying degrees in repelling a whole range of biting arthropods. Thus an effective repellent for Aedes mosquitoes is also broadly effective against other mosquito species, midges, fleas, sand flies, black flies, lice, ticks, bedbugs and leeches. Stinging insects like bees and wasps, however, are not affected.
Box 3 - Testing ... testing

The efficacy of insect repellents can be tested in many different ways. Entomologists use a variety of techniques, which leads to some difficulty in drawing conclusions from the results. The most convenient test is the cage test. An insect repellent is applied to one arm and both arms are thrust into a cage containing 20-40 hungry insects. The bites on each arm are counted and the experiment is repeated at hourly intervals. The results give a measure of how the repellent efficacy changes with time for a given biting insect. Many factors affect the results, and variations are found for different strains of the same species and for virtually every other experimental variable such as cage size, population density and insect age. The most important parameter which is obtained from testing is the length of time of complete protection.
A more realistic test is to enter a room containing 30 free flying hungry mosquitoes with just the legs exposed and count the bites for 10 minutes, then apply insect repellent to the legs and enter the room for 10 minutes each hour, counting the bites as before. After each exposure, the unprotected arms are exposed to collect any unfed mosquitoes and a new batch of mosquitoes introduced. The entomologists then do repellency testing outdoors which involves biting intensity measurements and insect identification.
Since rational molecular design criteria do not appear to be available, how else can suitable molecular targets be selected? Structure-activity studies do not give a clear answer and a visual examination of the structures (1)-(9) does not appear to reveal any common factors. Although two of the molecules have a 1,3-propanediol substructure, the other eight do not. Physical properties such as partition coefficient, molecular weight, infrared absorption, viscosity, surface tension, molecular polarisability and Hammett substituent constants have all failed to correlate with repellent activity.
One physical property, however, which must be crucial is vapour pressure: too high and repellency is soon lost as the substance vaporises from the skin, too low and the concentration available in the air is too low to have an effect. This can be verified for our small selection of compounds by calculating the vapour pressure at 37°C by using software freely available from the US Environmental Protection Agency.8 For a given chemical compound, entered by molecular structure (.mol file), Smiles string, chemical name or CAS Registry Number, a whole range of physical properties such as solubility, partition coefficient, degradation in air, water or soil, as well as vapour pressure, can be estimated. Some results are shown in Table 1. These confirm that citronellal is expected to be short acting and the more modern repellents towards the bottom of the table are likely to give much longer protection.
And finally
After years of recommending DEET as the only effective insect repellent, the US Centers for Disease Control and Prevention (CDC) issued a press release on 28 April 2005 entitled: CDC adopts new repellent guidance for upcoming mosquito season. The updated guidance includes addition of two active ingredients - picaridin and oil of lemon eucalyptus - which have been shown to offer long-lasting protection against mosquito bites.
Chris Cooksey can be contacted at dha@chriscooksey.demon.co.uk.
Further Reading
C. Peterson and J. Coats, Pesticide Outlook, 2001, 154.
G. Nentwig, Parasitol. Res., 2003, 90, S40.
References
- A. Blackwell et al, Scottish Crop Research Institute, Annual report 2002.
- L. Goodyer and R. H. Behrens, Am. J. Trop. Med. Hyg., 1998, 59 (2), 323.
- P. H. Knoess and E. G. Neeland, J. Chem. Educ., 1998, 75, 1267.
- P. Barbier and G. Leser, Comptes Rend., 1897, 124, 1308.
- H. E. Zimmermann and J. English, J. Am. Chem. Soc., 1953, 75, 2367; Y. Yoshifumi, H. Tsuruta and Y. Yuasa, Org. Proc. Res. and Dev., 2000, 4 (3), 159.
- B. L. Jensen, A. Malkawi and V. McGowan, J. Chem. Educ., 2000, 77, 1474.
- S. S. Basara et al, J. Med. Entomol., 2002, 39, 736.
- EPA






No comments yet