CPD and microscale chemistry are making practicals more possible and teachers more confident. Teacher trainer Robert Campbell explains how, then one of his PGCE students shares their first-hand experience of using microscale in their placement

Thanks to the pioneering work of Bob Worley, David Paterson and CLEAPSS, microscale chemistry is gaining interest among chemistry teachers and teacher educators, like me [Robert]. was keen to identify whether microscale resources could support new teachers to include more practical work in their teaching.
Does microscale mean more hands-on lessons?
More hands-on lessons?
I developed a research project working with early career teachers (ECTs) to investigate the utility of microscale practicals. The aim was to find out if these practicals supported ECTs to include a wider range of hands-on work in their teaching, and if so, how.
This introduction to microscale allayed their fears about the activities not being equivalent to the experimental method in examinations
The ECTs completed three Continuing Professional Development (CPD) sessions covering microscale practicals suitable for key stage 3 and 4 chemistry curriculums. Following each session, we gave the teachers class sets of microscale resources to trial in school and asked them to record reflective diaries exploring the advantages and limitations of these practicals. Their diary entries highlighted that they all found opportunities to include microscale within their teaching, and that microscale practicals could expose students to hands-on work and the underlying chemical concepts.
Do microscale practicals inform good practice?
Inform good practice?
Those initial reflections from the ECTs showed they liked the simplicity and efficiency of the microscale resources, particularly the microscale electrolysis practical. The resources are simply a laminated sheet with direct instructions and space for the learner to complete the work on (below).
ECTs acknowledged this meant the instructions were clear and concise. Moreover, the experimental setup developed opportunities to review other topics in chemistry. In the microscale electrolysis example, they particularly liked the inclusion of potassium bromide, potassium iodide and litmus paper, affording opportunities to discuss with learners why they were seeing the chemical reactions they were and to retrieve knowledge about displacement reactions or chemical tests for halogens such as chlorine.
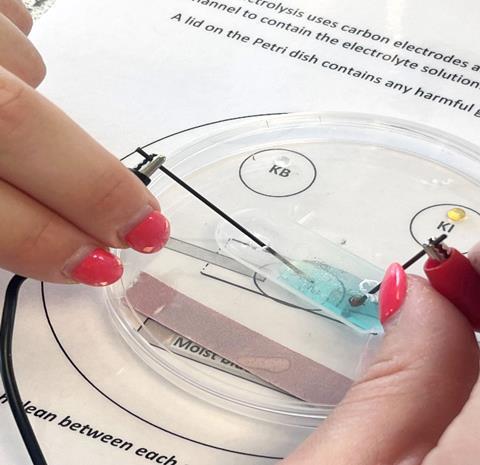
The final review of the project revealed that ECTs proactively included microscale practicals in teaching key stage 4 lessons. Since microscale experiments can be set up more efficiently than traditional practical work, they could apply the practical to exam-based questions within a single lesson. Equally, the flexibility of the microscale resources was advantageous. The ECTs told us they demonstrated a practical procedure to a whole class using a visualiser, ensuring that students understood how to complete the practical and its purpose. Perhaps surprisingly, this introduction to microscale resources allayed their fears about the microscale activities not being equivalent to the experimental method in core practical booklets or terminal GCSE examinations. They explained how discussions prompted by these microscale activities supported learners’ comprehension of challenging topics.
Can microscale help trainee teachers?
Given the enthusiasm the CPD engendered in the ECTs on the course, I then incorporated microscale activities in taught practical sessions on the PGCE science course at St Mary’s University. This gave pre-service science teachers access to both microscale and traditional large-scale practicals and led to them discussing and improving their understanding of the purpose of practical work. As a result, they have an improved understanding of chemical concepts and practical skills. After introducing these microscale practical sessions, I noticed more of these would-be teachers were including practical work in their lessons. And their feedback aligned closely with the experience of the early career teachers.
I’m now looking forward to including a wider portfolio of microscale practicals into my teaching on the PGCE course. However, I don’t believe this format should replace traditional practical work entirely. Ultimately though, careful consideration of when to use microscale practicals can foster strong useful skills in pre-service teachers alongside developing a deeper understanding of chemical concepts.

In the classroom with a trainee teacher
PGCE student, Bridget Eburne translated Robert’s introduction to microscale into a lesson on her teaching placement
Practicals are an essential part of science teaching, and the question ‘Are we doing a practical today, Miss?’ has been a mainstay of my teaching experience during my PGCE year. At both school and university, I often found traditional practicals challenging to complete due to complex methods and fragile equipment, and it was hard to link the practical experience back to the theory being taught alongside. So, when Robert Campbell first introduced me to microscale practicals as part of my training at St Mary’s University, explaining how it could help to avoid cognitive overload and maximise keeping learners’ minds-on work, I was excited to try it out at school.
When I set out to plan my lesson on displacement reactions for a GCSE chemistry class, numerous factors drew me to try a microscale practical with them. These students had not carried out many practicals before, so they had limited dexterity in manipulating the equipment and limited experience in keeping track of complex methods. The traditional approach to metal displacement has a high mental load, with similar-looking solutions needing to be combined sequentially to avoid contamination. This seemed like a good place to try microscale to minimise cognitive overload by providing scaffolding for these complex tasks. As well as considering the student perspective, I found the microscale approach appealing because it reduced the work for the science technicians.
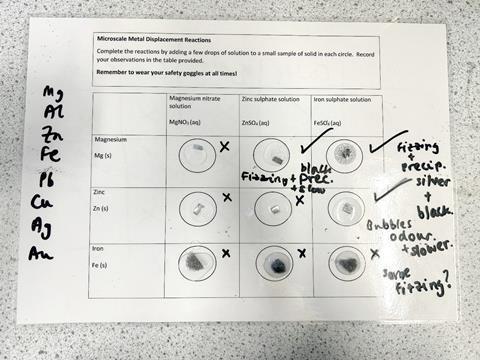
One immediate benefit of the microscale approach was when preparing students before the practical. I was able to use the class visualiser to demonstrate the method to them and to prompt discussion about what kind of observations they should be looking out for. The simplicity of the microscale setup made it easy to use with classroom technology. Consequently, it was easier for learners to engage with the displacement reaction practical. I also used a group AFL (Assessment for learning) activity to develop some hypotheses. Students could tick or cross each reaction with a whiteboard pen on the microscale laminate, to keep track of their expectations. This meant they could see the entire practical process of hypothesis, observation and comparison throughout the practical. It was a useful visual scaffold to promote more purposeful engagement.
Students were able to identify and fix their mistakes independently
Although the microscale approach enabled me to get students engaged and thinking critically about their work, the lesson was not without errors. The most common one was students mistaking one solution for another, leading them to complete reactions incorrectly. However, where a traditional practical would require new glassware and other fresh equipment, in this case they could simply wipe clean the laminate and I gave them very small volumes of extra reagents. Students were able to identify and fix their mistakes independently, leading to a significant boost in their confidence during this lesson.
Following the success of the metal displacement microscale practical with my year 10s, I would like to use this approach with classes who might have had fewer opportunities to develop fluency when working with practical equipment. Or those for whom the hands-on nature of traditional practicals can distract them from being minds on. I’d also like to enhance this by using the integrated instructions pioneered by David Paterson.









No comments yet