One of the most innovative developments to come from the discovery of carbon nanotubes is that of nanotube paper and thin films. 'Black paper' is now commercially available for the next generation of high-tech electronics

Black is the new white in paper. However, this paper is not for writing. Carbon nanotube paper represents a potential new phase for these materials as they find application in the development of flexible electrodes and batteries. In this article we discover how this is being done. But first we have to go back to the basics.
Back to basics
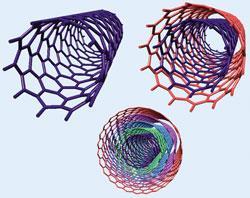
Carbon nanotubes consist of one or more concentric cylinders, which comprise hexagonal rings of carbon. They exist as single-walled carbon nanotubes (SWCNTs), multi-walled carbon nanotubes (MWCNTs) and, more recently, double-walled carbon nanotubes (DWCNT). But carbon nanotubes are unreactive, which makes them difficult to connect together and exploit in applications.
Initially scientists used high pressure techniques and ion beams to fuse the tubes together, and then turned their attention to modifying the surface of the nanotubes to create defects that could be further exploited. However, modifying the nanotube surface is not an easy process. There is a fine balance between adding functional groups and changing the outer layers of nanotubes, and thus altering its electronic and structural properties. The reason why carbon nanotubes exhibit such properties is because of the graphitic structure, specifically its sp2 bonding. A SWCNT is essentially a rolled-up graphene sheet, so any modification to this single layer could eliminate the properties we want.
One type of structural defect is a Stone-Wales defect, which is made up of two seven-membered rings and two five-membered rings, (1) and (2). Other defects may simply be pre-existing holes or tears on the sidewall of a tube. In 1998 Richard Smalley, professor of physics at Rice University in Houston, Texas, US, discovered that defects on the nanotubes could be oxidised to carboxyl groups by adding concentrated nitric acid, or a mixture of nitric and sulfuric acids to reduce the reaction time.
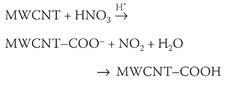
This reaction soon became the preferred method for exploiting the defect sites on carbon nanotubes. During the oxidation process, the nanotube structure is further disrupted resulting in additional holes and tears.
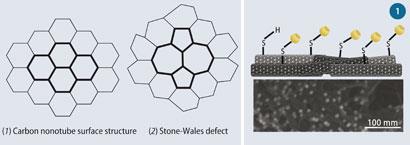
The nanotubes can also be modified by mechanical processes, such as ball-milling. In this process, MWCNTs are put into a ball-mill, which is degassed to prevent oxygen becoming a reactant. To add thiol groups, for example, to the surface of the nanotube, a constant supply of hydrogen sulfide is introduced into the vessel, which is sealed and ball-milled for a few days.1 After purification, thiolated MWCNTs (MWCNT-SH) are ready for use.
Mat formation
At Florida State University we have been looking at ways of covalently cross-linking carbon nanotubes. We wanted to find out why other researchers were having such difficulties doing this and, if we could overcome these difficulties, we hoped we would form mat-like structures for a host of new applications.
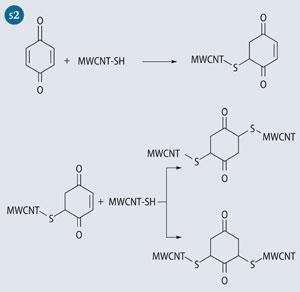
Our experimental work focused on covalently cross-linking MWCNT-SH using benzoquione following the Michael addition pathway (see Scheme 1). The original idea came from cross-linking techniques used in biochemistry to attach maleimide-based molecular labels to the amino acid cysteine.
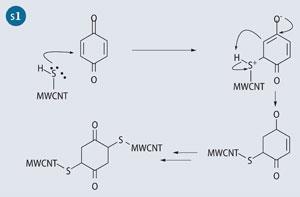
A closer look at benzoquinone shows that there are two possible positions of reactivity on each C=C bond, resulting in the two adducts with respect to the thiolated positions on the quinone. The 2,5-dithioMWCNT-1,4-cyclohexanedione product may occur when steric interactions from the nanotube govern the chemical reaction though if two nanotubes were to orient themselves parallel or end-on, then the 2,6-dithioMWCNT 1,4-cyclohexanedione configuration may dominate (see Scheme 2).2 Fortunately, for the purposes of cross-linking, either product is beneficial.
Our method of nanotube mat formation is a modified filtration-from-suspension (FFS) technique whereby the nanotubes are heated in a reaction flask with the cross-linker then poured into a vacuum filtration funnel. We control the temperature by using a heat gun, which as well as heating the reaction also encourages the cross-linking process. The average diameter of the MWCNT-SH we use is 9.5 nm with the lengths typically <1 μm. The degree of coverage by thiol groups over the surface of the nanotubes is between 0.5 and 1 per cent.
With the right concentration of cross-linker, we can produce a flexible mat, the thickness of which is controlled simply by the diameter of the filtration funnel, and we typically achieve between 80 and 270 μm in thickness across the mat. We also find that a concentration ratio of 5:1 (benzoquinone : MWCNT-SH by weight) is optimum in producing flexible mats. Higher concentrations of cross-linkers result in over cross-linking and brittle mats. The 5:1 mat can be folded just like regular paper and even rolled up.
As an added benefit of our technique, we can attach various sizes of gold nanocrystal (up to 13 nm) to the un-reacted thiol groups on the surface of the nanotube mat (see Fig 1). This could potentially lead to tailored functional nanocrystals, such as quantum dots, on the surface of the mats which could be used in such applications as photovoltaics.
Of interest, vertically aligned nanotubes, which are used to make carbon nanotube paper, are revealing new properties. In some cases, for example, these mats can absorb 97-99 per cent of the wavelength of light, resulting in one of the blackest materials known aside from a black body (which absorbs all electromagnetic radiation).3
Buckypaper link
Our success in making cross-linked mats is quite remarkable since to-date the closest match for this type of nanotube paper, the so-called 'buckypaper', from SWNCTs and DWCNTs, has required high pressure techniques and ion beams to fuse the tubes together. (Ion beam fusing is the bombardment of carbon nanotubes with species such as CF3+ that cause functionalisation, defects and cross-linking with nanotubes.) Other researchers have produced buckypaper by filtering SWCNTs with surfactants, such as Triton X-100 - 4-(1,1,3,3-tetramethylbutyl)phenyl-polyethylene glycol.
Buckypaper is commercially available and typically costs around $200 for a 40 mm diameter sample made from SWCNTs or DWCNTs.4 With the cost for production of carbon nanotubes falling, there will soon be highly competitive rates for buckypaper and other nanotube-based advanced materials.

Ben Wang, director of the High-Performance Materials Institute in the US and professor in the industrial and manufacturing engineering department at Florida State University, US, is currently exploring different methods of manufacturing nanotube composites for use in applications, ranging from body armour to flat-panel displays. In addition to processing buckypaper, the team is also exploring the effect of surfactants and nanotube alignment on the conductive and mechanical properties of buckypaper. To-date the researchers have found that both nanotube alignment and resins can improve the characteristics of CNT thin films. Their findings are attracting much attention in the engineering community to the extent that companies such as AT&T, Boeing, BP, Lockheed Martin, Motorola, and Sun Microsystems are now sponsoring their research. Wang's latest nanotube project, Nanotubes optimised for lightweight exceptional strength (NOLES), is set to receive $3.2m from congressional funding in 2010.
Potential of thin-film CNTs

Developments in past year have also put us one step closer to other applications of thin-film CNTs. For example, researchers at the University of Texas, are developing nanotube-based muscles that could potentially be used as human implants in place of destroyed muscle or in small electrical devices that require muscle-like movement. The team at Texas has developed carbon nanotube aerogel sheets from forests of multi-walled carbon nanotubes.5 The sheets behave as rubber when stretched, which is an important feature for movement. The actual process of actuation occurs when a positive voltage is applied to the nanotube sheet electrode, with respect to a counter electrode.
Nanotube films have also been in development for the past 10 years, the difference between these films and buckypaper residing in the type of preparation and inclusion of polymers. Unidym, a company based in California, US, is one of the first companies to produce nanotube films. These conductive sheets have the potential to be used in applications such as LCD screens which Unidym has already demonstrated as a prototype in collaboration with Silicon Display Technology based in Seoul, Korea. Other applications include e-books and flexible thin-film solar cells, an area of research in the Kroto research group at Florida State University.
However, as with many developments in science, potential of carbon nanotubes to enhance our way of life, may come with a price. Recently health and safety concerns have arisen about the size of these tubes and their similarities to asbestos. Although there has yet to be a case where carbon nanotubes have caused mesothelioma (a type of cancer that is mostly attributed to long-term exposure to asbestos) in people, the fear is that we may be entering a phase where the development of new materials containing nanotubes supersedes the research into their health and safety. Fortunately the Environmental Protection Agency (EPA) has now started a scheme whereby businesses can register and submit information about nanomaterials in their products to help provide a 'scientific foundation for regulation'.6 This is a step in the right direction even if involvement is on a voluntary basis.
While research into carbon nanotube-based systems is progressing well, there is a new carbon compound on the block causing a stir in research labs. Graphene is a single layer of sp2-bonded carbon, and many of the applications currently being considered with CNTs would also be applicable to graphene with the advantage that the entire structure of the latter is available to react. However, it remains to been seen whether graphene will elicit as much interest as carbon nanotubes.
Steve Acquah is a postdoctoral associate and director of GEOSET at Florida State University, 95 Chieftan Way, Tallahassee, Florida 32306, and can be contacted at: sacquah@chem.fsu.edu. Darryl Ventura is a doctoral student working in the department of chemistry, and Sir Harold Kroto is Francis Epps professor of chemistry at the same institution.
References
- Z. Konyaet al, Chem. Phys. Lett., 2002, 360, 429.
- D. N. Ventura et al, Carbon, 2010, 48, 987.
- K. Mizuno et al, PNAS, 2009, 106 (15), 6044.
- NanoLab, Newton MA 02458, US.
- A. E. Aliev et al, Science, 2009, 323, 1575.
- US Environmental Protection Agency Office of Pollution Prevention and Toxics: Nanoscale materials stewardship program - interim report, 2009.






No comments yet