By 2030, the world's population is expected to rise from the current 6.7 billion to over eight billion. As a consequence much agricultural land will be lost under cities and roads. This, coupled with economic growth across Asia, and a renewed focus on biofuels, is putting increasing pressure on food supplies and a growing demand for higher crop yields. The need for safe and environmentally-friendly crop protection chemicals has never been greater.
-
Crop yields would be around half their current levels without the use of herbicides, insecticides and fungicides

About 800 chemicals are registered for use in crop protection around the world, representing a major $multi-billion industry. Several global companies make it their business to discover, develop and sell new products, one of which can cost the company ca $200m to bring to the market. A typical research programme may take five years, and this is followed by a development period of around eight years, during which time the chemical will be rigorously tested to ensure that it meets the highest standards of safety - to farmers, consumers and in the environment. In addition, the route by which the compound will be prepared on a multi-tonne scale is determined during this development period. But are these chemicals the answer to food sustainability and what is the outlook for the development of new products?
The benefits of crop protection chemicals
Crop protection chemicals provide farmers with a cost-effective way of improving the yield and the quality of their crops. They also make harvesting more straightforward and maintain consistent yields from year to year.
The main classes of crop protection chemicals are herbicides, insecticides and fungicides. Selective herbicides, for example, control the growth of weeds which would otherwise grow among a crop, competing with it for water, nutrients and sunlight. Without crop protection chemicals agriculture would be less efficient.
Research done over the past 20 years, mainly by Dr Erich-Christian Oerke and his colleagues at the University of Bonn in Germany, has shown that overall crop yields would be around half their current levels without the use of crop protection products. In certain crops, for example cotton, which can be spoilt by a host of different insects and competing weeds, the losses can be as high as 80 per cent.1
Research has also been done by other groups to compare yields obtained from crops grown organically, where the use of crop protection products is minimised. (Generally no synthetic crop protection chemicals are used, though minerals, such as copper salts, and natural chemicals, such as the insecticide rotenone found in the roots of several plants, may be.) The findings suggest that organic yields are usually well below those achieved when crop protection chemicals are used, typically 30 per cent lower for wheat and barley, and 40 per cent lower for potatoes.2
Moreover entire crops have been lost without the use of crop protection chemicals. Potatoes and vines are examples of particularly high-risk crops, growing in parts of the world where weather conditions can at times be conducive to fungal epidemics. The devastation of entire crops is of major concern, both to farmers, who lose income, and to consumers, who face rising supermarket prices.
Modern crop protection chemicals
Inorganic compounds, such as arsenic and mercury salts, were used in agriculture until the 19th century to control insects and fungi, respectively. These practices continued until the 1930s, when simple synthetic organic chemicals, eg the herbicide 2,4-D (dichlorophenoxy ethanoic acid) and the insecticide DDT (dichlorodiphenyltrichloroethane) came on the market.
Crop protection chemistry has come a long way since then. Modern products are designed to be highly selective in their action to minimise the impact on non-target organisms. Selective activity enables, for example, the control of fungi growing on plants without damaging the plants themselves; or the control of a range of weed species without damaging the crop plants among which the weeds are growing. An essential aspect of the selectivity of a crop protection chemical is that it does not affect the consumer or the environment. Each of these effects may stem from specificity at the enzyme or receptor level, or from selective metabolism.
There has also been a trend towards the use of lower application rates for these chemicals, though this can really only be seen by looking back over decades rather than years. For example, the herbicide 2,4-D is typically applied at rates of about 1000g ha-1, while the best of the modern herbicides such as the sulfonylureas are effective at rates as low as 10g ha-1.
Modern compounds are also safer than their older counterparts, and the hurdles for registration are higher than in the past. Residual levels in food are measured to ensure they do not pose a health risk, and their persistence in the environment and bioaccumulation are also determined.
Herbicides
The non-selective herbicide glyphosate (1) was introduced by Monsanto in 1973. This herbicide controls the growth of a wide variety of plants, both weeds and crops, and compounds of this type can be used as an alternative to ploughing. Glyphosate was discovered during a chemistry-led research programme in which more than 100 novel aminomethylphosphonic acids were synthesised and tested for interesting properties in a variety of screens. Farmers use glyphosate when they want to clear all the plants from a field. The herbicide kills even perennial plants, since it can move from the leaf, where the spray droplets fall, into the root.
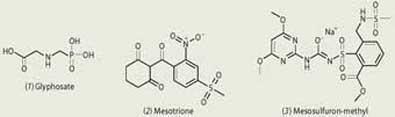
Glyphosate inhibits 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS), an enzyme involved in the biosynthesis of aromatic amino acids, which are required for protein biosynthesis and thus plant growth. This enzyme is absent in mammals (which rely on plants in their diet for aromatic amino acids), so glyphosate has little toxicity. The compound is also safe in the environment, since it binds to soil and degrades quickly.
Mesotrione (2), a Syngenta product, is one of a family of herbicides which inhibit 4-hydroxyphenylpyruvate dioxygenase (HPPD), an enzyme on the biochemical pathway that leads to plastoquinone, a co-factor in carotenoid biosynthesis. (Carotenoids are essential pigments in plants which harvest light energy for growth and protect the plant from photodamage.) Mesotrione is a selective herbicide, providing post-emergence control of broad-leaf weeds in maize, and is often used in mixtures with other herbicides to take advantage of dual modes of action.
Mesosulfuron-methyl (3) was developed by chemists at Bayer CropScience and is a member of the sulfonylurea family of herbicides that act by inhibiting the enzyme acetolactate synthase (ALS), which catalyses a step in the biosynthesis of branched-chain amino acids. It is used for the post-emergence control of grasses and some broad-leaf weeds in cereal crops at rates as low as 15g ha-1.
Fungicides
Azoxystrobin (4), the world's largest-selling fungicide, was discovered and developed by Syngenta chemists. A broad-spectrum member of the strobilurin family, this fungicide acts by inhibiting mitochondrial respiration in fungi by binding at a specific site on cytochrome b, and thereby blocking the production of adenosine triphosphate (ATP) and suppressing the energy cycle.
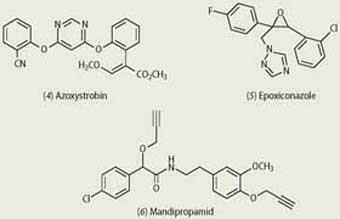
The azoles, compounds containing a 1,2,4-triazole (or, in a few cases, an imidazole) ring, are another important class of fungicide. They act by inhibiting the biosynthesis of ergosterol, an important component of fungal cell walls. Epoxiconazole (5), developed by BASF, is one of the leading compounds of this class. It has a broad spectrum of preventative and curative activity and is used, for example, in cereals, sugar beet, peanuts and oilseed rape.
Mandipropamid (6), a mandelic acid amide fungicide, was launched by Syngenta in 2007. It is used to prevent damage caused by various fungal diseases such as downy mildew on grapes and late blight on potatoes.
Insecticides
The pyrethroids are a major class of insecticides, and have been in use for over 25 years. Their synthesis was inspired by the natural pyrethrins. λ-Cyhalothrin (7) is used for the control of insects in a range of crops, including cereals, cotton and potatoes, and works by disrupting the central nervous system of insects. The chemical interferes with the function of membrane-spanning sodium channels, leading ultimately to paralysis and death.
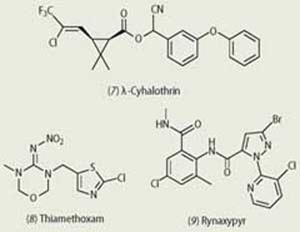
Thiamethoxam (8) (Syngenta) is an insecticide of the neonicotinoid family, first sold in 1997, and used to control aphids, whiteflies, thrips, hoppers and other insect pests in a wide variety of crops, including rice, soybean, cereals, sugar beet and cotton. Again, it interferes with the nervous system of insects but, in this case, is an agonist of the nicotinic acetylcholine receptor.
Rynaxypyr (9), a new insecticide from DuPont, has recently received approval for use in the US and Canada. It selectively blocks calcium ion channels crucial for muscle function in insects. It will be used in a variety of fruit and vegetable crops, including apples, grapes, tomatoes and lettuce.
Growing resistance

Despite the availability of so many chemicals for crop protection, there is always scope to replace some of the older products with better ones, especially those where resistance is becoming a problem.
The onset of resistance, though difficult to predict, is a feature of modern compounds with a specific biochemical mode of action. Small changes in the target organism, at the target site for example, render the compounds ineffective.
The broad-spectrum herbicide glyphosate is the world's largest-selling crop protection chemical. It is used in partnership with maize and soya which have been genetically engineered to be resistant to its effects. However, after many years of extensive use, in 1996, several species of glyphosate-resistant weeds emerged, followed by others from many parts of the world. So glyphosate will ultimately need a replacement and the discovery of such a compound will be a glittering prize for any crop protection company.
Another driver for the search for new and improved products are the re-registration programmes currently going on in various regions of the world, including Europe. These programmes are aimed at ensuring that all crop protection chemicals meet modern safety and environmental standards. The re-registration procedure is expensive, and the cost has to be met by the manufacturers. As a consequence, some of the smaller products are being lost to the farmer because their sales don't justify the costs associated with re-registration.
In search of new crop protection chemicals
Chemists often work for long periods, sometimes years, in their search for new products, without discovering the one compound they are looking for that meets all of the following criteria:
-
high potency against a suitable spectrum of weeds, insects or fungi;
-
high selectivity, allowing the compound to be applied at low rates and without affecting non-target organisms;
-
chemical and metabolic stability, so that it survives in sunlight on the surface of a leaf, and then during translocation to its biochemical target within the plant, but without being so stable that it persists in the environment.
As a consequence, successful projects have to lead to highly profitable new products which pay not only for themselves but also for all the unsuccessful research.
The research involves the synthesis and biological screening of many compounds. However, new technologies, especially advances in robotics, automation and data-handling, have dramatically changed the way in which a research project is done. For example, preliminary screens have been miniaturised, and test chemicals can now be applied to plants, fungi and insects growing in 96-well microtitre plates. The plates are bar-coded and handled robotically, and the test chemicals, now required only in milligram quantities, are sprayed into the wells by computer-controlled instruments.
Chemists are also able to use automation to make sets of related compounds in parallel using the same synthetic steps, sometimes hundreds at a time, by 'combinatorial chemistry' or 'parallel synthesis'. In fact, it is not difficult to make large numbers of compounds, the real skill is to design sets of compounds with diverse structures, and properties which are likely to lead to biological activity. Combinatorial chemistry has not been greatly successful as a way to generate new leads, but it is used to make targeted sets of chemicals more efficiently during optimisation programmes.
Nature is a rich source of biologically active compounds. Many plants, for example, produce insecticides to protect themselves, and micro-organisms are also prolific producers of bioactive natural products. Natural products are important to the crop protection industry because they often have novel structures and modes of action. However, they do not usually have the properties required for a crop protection chemical. Poor photostability, for example, is sometimes a problem. Instead, they serve as inspiration for programmes of synthesis, which lead to structurally related compounds, tailored to the properties required by a crop protection chemical.
Finally, every company carefully monitors its competitors' patents to identify new areas of interest, looking for loopholes that can be exploited. Although this approach is probably the most reliable way to find biological activity, it is not without its problems. By definition, the competitor is ahead of the game and, before long, several companies are likely to be working on closely-related chemistry and racing each other to the patent office.
Outlook
The world has recently had a wake-up call over food shortages, and climate change will also present agriculture with future challenges. People now realise how precarious the balance is between supply and demand. Crop protection chemicals have an important contribution to make to the global challenge of producing more food from the same area of farming land.
Sarah Armstrong and John Clough are research chemists working for Syngenta based at the Jealott's Hill research station, Bracknell, Berks SL6 42Y.
Further reading
- K. Beautement et al, Pest. Sci., 1991, 31, 499-519
- J. M. Clough and C. R. A. Godfrey in Agrochemicals and plant protection, D. H. Hutson and J. Miyamoto (eds), chap 5, pp 109-148. Chichester: Wiley, 1998
- H. Sauter, W. Steglich and T. Anke, Angew. Chem. Int. Edn, 1999, 38, 1328-1349
Certain species of Basidiomycete fungi that grow on rotting wood produce compounds with fungicidal activity. One example is Oudemansiella mucida, a European fungus that grows on the beech tree. In the 1960s and 1970s Czech and German chemists independently isolated and characterised a family of fungicides from this fungus. Two of the compounds - strobilurin A and oudemansin A - caught the attention of ICI chemists (now Syngenta) in the early 1980s. The natural products were attractive because of their relatively simple structures and bioactivity, and also because their fungicidal effects were shown to stem from what was then a novel mode of action, ie inhibition of mitochondrial respiration by binding at a site on cytochrome b for which inhibitors had not previously been described.

Oudemansin A proved to have a useful spectrum of activity against fungi growing on plants in the glasshouse, though it lacked potency. By contrast, strobilurin A was not active in the glasshouse, and the chemists showed that this was the result of its photochemical instability, which meant that it rapidly broke down on the surface of a leaf.
Taking a lead from NatureBased on their knowledge of the structures, activity and mode of action of the natural products, the chemists set about synthesising the two compounds. Their goal was to find synthetic analogues with higher levels of fungicidal activity and suitable stability for application as foliar sprays. They were also looking for analogues that could move through the plant, an important property of modern fungicides which improves field performance by redistribution of the compound within plant tissue after spraying. They reasoned that the trans -methyl 3-methoxypropenoate group was likely to be required for activity, so this was retained in the various analogues that were prepared, while the carbon chain at the 2-position was modified in various ways. About 1400 analogues were prepared and tested.
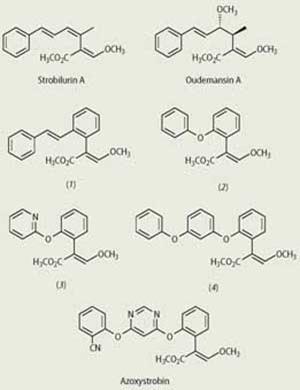
The chemists discovered that the cis -alkenic bond of strobilurin A could be replaced by an ortho -disubstituted benzene ring to give the stilbene (1), which is more stable in light than the natural product. The stilbene was active in the glasshouse but degraded too quickly in light to be useful in the field. The diphenyl ether (2) was more stable in light and was active in the field, albeit at rather high application rates. Furthermore, (2) was systemic in plants. Modifications to (2) led to the analogues (3) and (4). The tricyclic derivative (4) had improved levels of fungicidal activity, but was too lipophilic to be able to move through the plant. The pyridine (3) retained the activity of its isostere (2) - isosteres are molecules, or functional groups, with similar shapes but different physical properties. As expected, (3) was highly mobile (probably too mobile) in plants. A combination of the ideas which led from (2) to (3) and (4), together with a huge amount of experimentation, led finally to the discovery of azoxystrobin, which was first prepared in 1988 and launched on the market in 1996.
Meanwhile BASF, whose chemists had been working independently on the strobilurins, also launched its first product in 1996. Soon other companies realised the importance of the strobilurins and began their own research programmes in the area. There is now a family of commercial strobilurins from different companies, but azoxystrobin is the biggest selling strobilurin and, indeed, the world's biggest selling agricultural fungicide, with sales well in excess of $500 million per annum.
References
1. E. -C. Oerke et al, J. Agricult. Sci., 2006, 144, 31-43.
2. See, for example, H. G. Hewitt, Outlooks on Pest Management, 2004, 15, 90-95.









No comments yet